|
CVnCoV
The CureVac COVID-19 vaccine (abbreviated CVnCoV) was a COVID-19 vaccine candidate developed by CureVac, CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline. Efficacy On 16 June 2021, CureVac said its vaccine showed 47% efficacy from its Phase IIb/III trial. Later, the final result data showed an efficacy of 48% against symptomatic disease in all age groups and, for people aged 18 to 60 years, an efficacy of 53% against symptomatic disease, 77% against moderate and severe disease and 100% against hospitalization and death, as no cases were detected in the study. This was based on interim analysis of 134 COVID cases in its Phase III study conducted in Europe and Latin America. The final analysis for the trials requires a mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CureVac
CureVac N.V. is a German biopharmaceutical company that develops therapies based on messenger RNA (mRNA). Legally domiciled in the Netherlands and headquartered in Tübingen, Germany, the company was founded in 2000 by Ingmar Hoerr (CEO), Steve Pascolo (CSO), Florian von der Mulbe (COO), Günther Jung, and Hans-Georg Rammensee. CureVac had approximately 240 employees in November 2015 and 375 in May 2018. At the beginning of the COVID-19 pandemic, CureVac was considered a beacon of hope for the development of a German vaccine. The federal government invested 300 million euros in the company. First, the CureVac vaccine was delayed due to minor problems, while the previously unknown BioNTech developed with Pfizer the very effective BNT162b2. In mid-2021, however, it became clear that the CureVac-vaccine was only 47 percent effective and is far away from approval. The company's focus is on developing vaccines for infectious diseases and drugs to treat cancer and rare diseases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Vaccine
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 ( COVID19). Prior to the COVID19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). This knowledge accelerated the development of various vaccine platforms during early 2020. The initial focus of SARS-CoV-2 vaccines was on preventing symptomatic, often severe illness. In January 2020, the SARS-CoV-2 genetic sequence data was shared through GISAID, and by March 2020, the global pharmaceutical industry announced a major commitment to address COVID19. In 2020, the first COVID19 vaccines were developed and made available to the public through emergency authorizations and conditional approvals. Initially, most COVID19 vaccines were two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Severe Acute Respiratory Syndrome Coronavirus 2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a Novel coronavirus, provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called the human coronavirus 2019 (HCoV-19 or hCoV-19). First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020. SARS‑CoV‑2 is a positive-sense single-stranded RNA virus that is Contagious disease, contagious in humans. SARS‑CoV‑2 is a virus of the species ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV), related to the Severe acute respiratory syndrome coronavirus 1, SARS-CoV-1 virus that caused the 2002–2004 SARS outbreak. Despite its close relation to SARS-CoV-1, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultracold Freezer
An ultra low temperature (ULT) freezer is a refrigerator that stores contents at between . An ultra low temperature freezer is commonly referred to as a "minus 80 freezer" or a "negative 80 freezer", referring to the most common temperature standard. ULT freezers come in upright and chest freezer formats. Application In contrast to short term sample storage at by using standard refrigerators or freezers, many molecular biology or life science laboratories need long-term cryopreservation (including "cold chain" and/or " colder chain" infrastructures) for biological samples like DNA, RNA, proteins, cell extracts, or reagents. To reduce the risk of sample damage, these types of samples need extremely low temperatures of . Mammalian cells are often stored in dewars containing liquid nitrogen at . Cryogenic chest freezers can achieve temperatures down to , and may include a liquid nitrogen backup. Biological samples in ULT freezers are often stored in polymer tubes and microtubes, g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
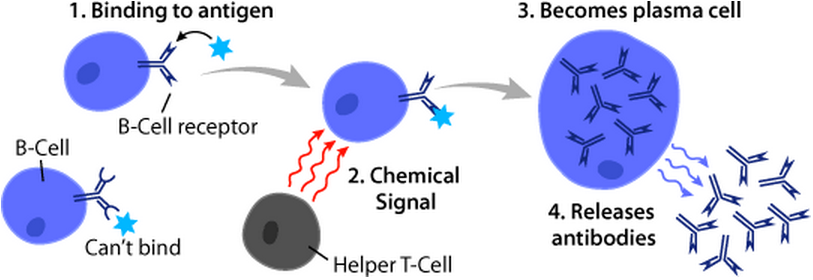
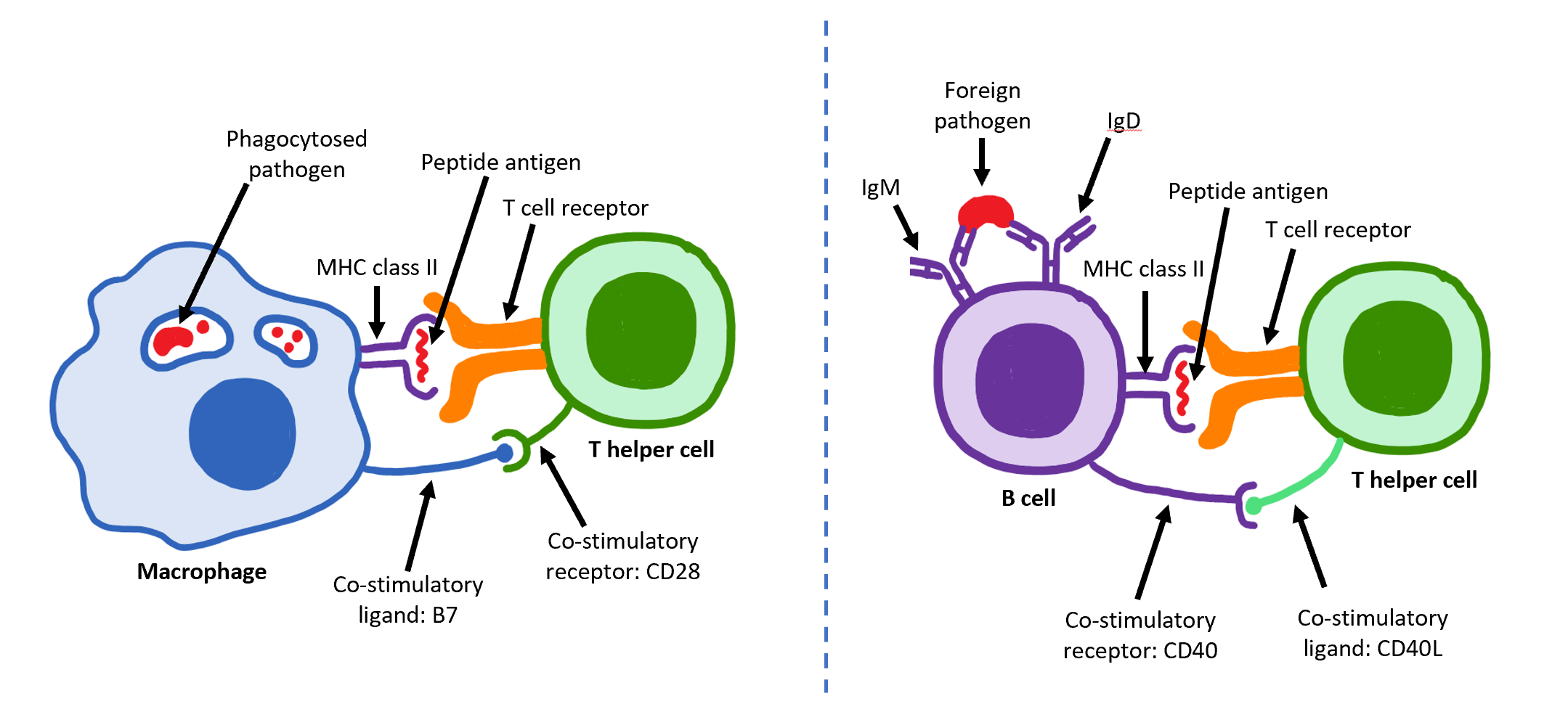
B Cell
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted or inserted into the plasma membrane where they serve as a part of B-cell receptors. When a naïve or memory B cell is activated by an antigen, it proliferates and differentiates into an antibody-secreting effector cell, known as a plasmablast or plasma cell. Additionally, B cells present antigens (they are also classified as professional antigen-presenting cells (APCs)) and secrete cytokines. In mammals, B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, which is why the 'B' stands for bursa and not bone marrow as commonly believed. B cells, unlike the other two classes of lymphocytes, T cells and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T Helper Cell
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases. Structure and function Th cells contain and release cytokines to aid other immune cells. Cytokines are small protein mediators that alter the behavior of target cells that express receptors for those cytokines. These cells help polarize the immune response depending on the nature of the immunological insult ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interferon
Interferons (IFNs, ) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses. IFNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens. Interferons are named for their ability to "interfere" with viral replication by protecting cells from virus infections. However, virus-encoded genetic elements have the ability to antagonize the IFN response contributing to viral pathogenesis and viral diseases. IFNs also have various other functions: they activate immune cells, such as natural killer cells and macrophages, and they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex (M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convalescence
Convalescence is the gradual recovery of health and strength after illness or injury. It refers to the later stage of an infectious disease or illness when the patient recovers and returns to previous health, but may continue to be a source of infection to others even if feeling better. In this sense, "Healing, recovery" can be considered a synonymous term. This also sometimes includes Patient Care, patient care after a major surgery, under which they are required to visit the Physician, doctor for regular check-ups. Convalescent care facilities are sometimes recognized by the acronym TCF (Transitional Convalescent Facilities). See also * Drug rehabilitation, Rehabilitation, therapy to control a medical condition such as an addiction * Recuperation (recovery), a period of physical or mental recovery * Recuperation (sociology), a sociological concept * Relapse, reappearance of symptoms * Remission (medicine), Remission, absence of symptoms in chronic diseases References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutralizing Antibody
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy. Mechanism In order to enter cells, pathogens, such as circulating viral particles or extracellular bacteria, use molecules on their surfaces to interact with the cell surface receptors of their target cell which allows them to enter the cell and start their replication cycle. Neutralizing antibodies can inhibit infectivity by binding to the pathogen and blocking the molecules needed for cell entry. This can be due to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moderna COVID-19 Vaccine
The Moderna COVID19 vaccine (INN: elasomeran), sold under the brand name Spikevax, is a COVID-19 vaccine developed by American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). Depending on the jurisdiction, it is authorized for use in people aged six months, twelve years, or eighteen years and older. It provides protection against COVID-19 which is caused by infection by the SARS-CoV-2 virus. It is designed to be administered as two or three 0.5 mL doses given by intramuscular injection at an interval of at least 28 days apart. It is an mRNA vaccine composed of nucleoside-modified mRNA (modRNA) encoding a spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. It is authorized for use at some level in many countries. In August and September 2022, bivalent versions of the vaccine (Moderna COVID-19 Vaccine, Bivalent) containing elasome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
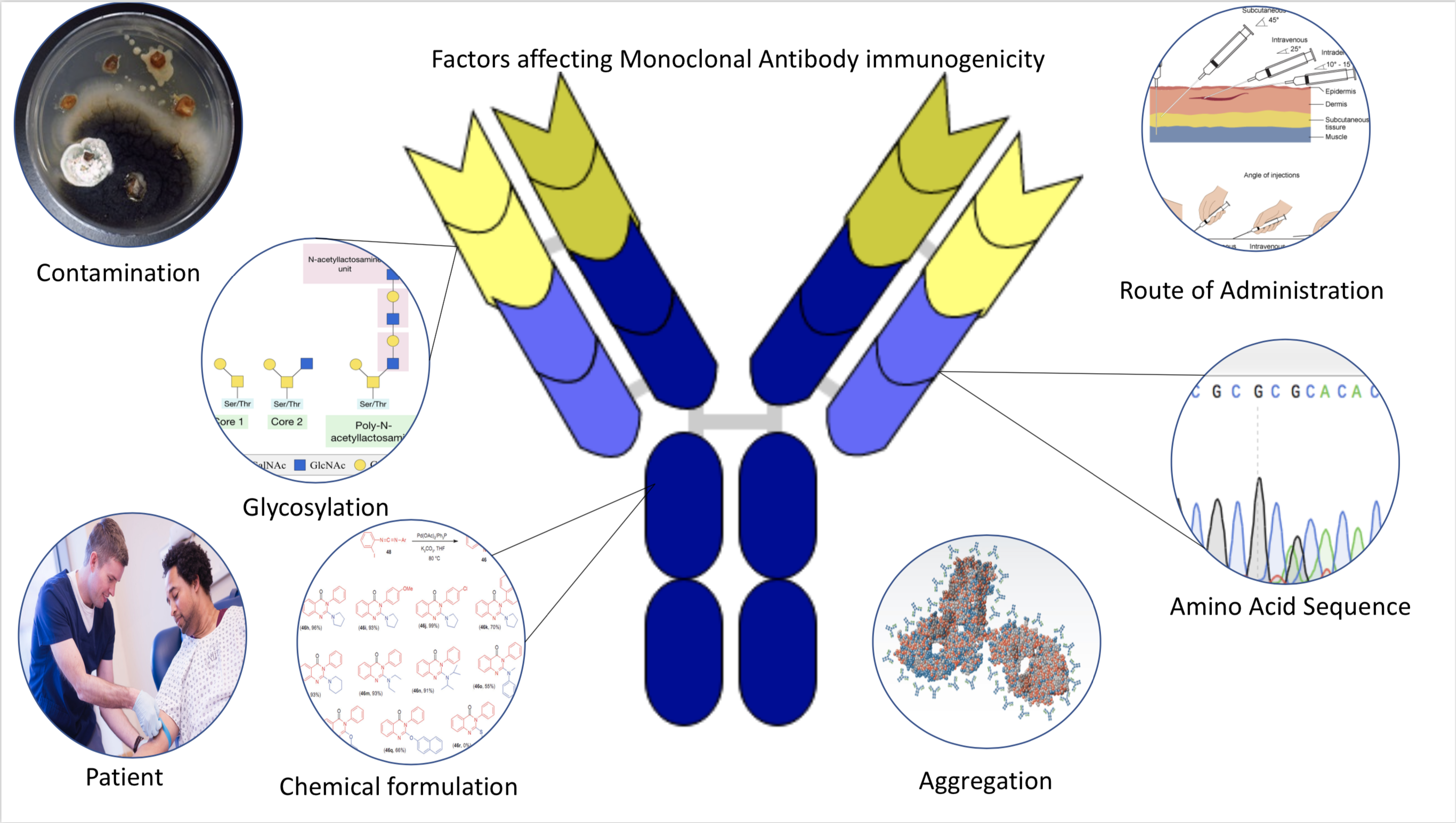
Immunogenicity
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)