|
CNN2
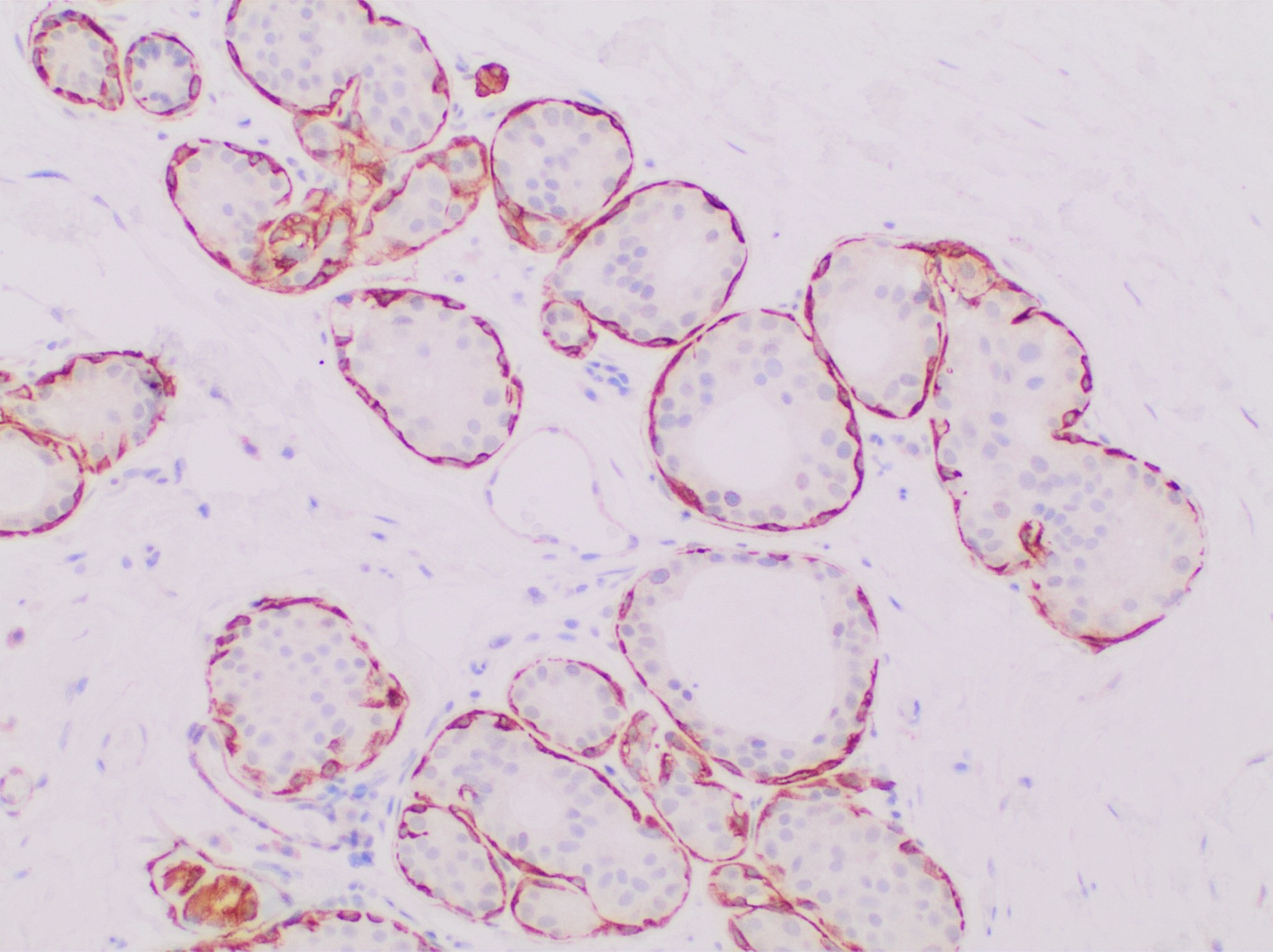
Calponin 2 is a protein that in humans is encoded by the ''CNN2'' gene. The ''CNN2'' gene is located at 19p13.3 in the human chromosomal genome, encoding the protein calponin 2. Calponin 2 is one of the three isoforms of calponin and an actin filament-associated regulatory protein with wide tissue distributions. Human calponin 2 is a 33.7-kDa protein consisting of 309 amino acids with an isoelectric point (pI) of 7.23. Accordingly, it is also known as neutral calponin. Evolution Calponin isoforms are conserved proteins whereas calponin 2 has diverged from calponin 1 and calponin 3 mainly in the C-terminal variable region. Phylogenetic lineage of calponin 2 showed that calponin 2 is conserved among mammalian species but more diverged among amphibian, reptile and fish species (Fig 1). Tissue distribution ''CNN2'' is expressed in a broader range of tissue and cell types, including developing and remodeling smooth muscle as well as adult mature smooth muscle, epidermal kera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin 3, Acidic
Calponin 3. acidic is a protein that in humans is encoded by the ''CNN3'' gene. The ''CNN3'' gene is located at 1p22-p21 in the human chromosomal genome. ''CNN3'' gene contains 7 exons and encodes calponin 3, a 36.4-kDa protein consisting of 329 amino acids with isoelectric point (pI) of 5.84. Calponin 3 is known as acidic calponin. Among three isoforms of calponin, less is known for the gene regulation and function of calponin 3. Nonetheless, much has been learned from extensive studies on the homologous genes ''CNN1'' and ''CNN2'' that encode calponin 1 and calponin 2. Evolution ''CNN3'' is one of the three homologous calponin isoform genes. Calponin 3 is significantly diverged from calponin 1 and calponin 2 in the C terminal variable region. The higher degree of divergence among vertebrate ''CNN3'' genes than that in the ''CNN1'' and ''CNN2'' gene families suggests possibly earlier emergence of ''CNN3'', indicating that calponin 3 may represent a prototype of calponin ances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNN2 Wikipage
CNN (Cable News Network) is a multinational cable news channel headquartered in Atlanta, Georgia, U.S. Founded in 1980 by American media proprietor Ted Turner and Reese Schonfeld as a 24-hour cable news channel, and presently owned by the Manhattan-based media conglomerate Warner Bros. Discovery, CNN was the first television channel to provide 24-hour news coverage and the first all-news television channel in the United States. As of September 2018, CNN had 90.1 million television households as subscribers (97.7% of households with cable). According to Nielsen, in June 2021 CNN ranked third in viewership among cable news networks, behind Fox News and MSNBC, averaging 580,000 viewers throughout the day, down 49% from a year earlier, amid sharp declines in viewers across all cable news networks. While CNN ranked 14th among all basic cable networks in 2019, then jumped to 7th during a major surge for the three largest cable news networks (completing a rankings streak of Fox N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin Interacted Proteins Wikigene Fianl
Calponin is a calcium binding protein. Calponin tonically inhibits the ATPase activity of myosin in smooth muscle. Phosphorylation of calponin by a protein kinase, which is dependent upon calcium binding to calmodulin, releases the calponin's inhibition of the smooth muscle ATPase. Structure and function Calponin is mainly made up of α-helices with hydrogen bond turns. It is a binding protein and is made up of three domains. These domains in order of appearance are Calponin Homology (CH), regulatory domain (RD), and Click-23, domain that contains the calponin repeats. At the CH domain calponin binds to α-actin and filamin and binds to actin within the RD domain. Calmodulin, when activated by calcium may bind weakly to the CH domain and inhibit calponin binding with α-actin. Calponin is responsible for binding many actin binding proteins, phospholipids, and regulates the actin/myosin interaction. Calponin is also thought to negatively affect the bone making process due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in '' Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene (journal)
''Gene'' is a peer-reviewed scientific journal in genetics and molecular biology, focusing on the cloning, structure, function, as well as the biomedical and biotechnological importance of genes. It was established in 1976 and is published by Elsevier. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 3.688. Beyond Gene, there are several sub-specialty journals linked to Gene including Meta Gene, Plant Gene, Agri Gene and Gene Reports. References External links * Genetics journals Elsevier academic journals Publications established in 1976 English-language journals {{genetics-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinin
Actinin is a microfilament protein. Alpha-actinin-1 is necessary for the attachment of actin myofilaments to the Z-lines in skeletal muscle cells, and to the dense bodies in smooth muscle cells. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. The non-sarcomeric alpha-actinins, encoded by ''ACTN1'' and ''ACTN4'', are widely expressed. ''ACTN2'' expression is found in both cardiac and skeletal muscle, whereas ''ACTN3'' is limited to the latter. Both ends of the rod-shaped alpha-actinin dimer contain actin-binding domains. Mutations in ''ACTN4'' can cause the kidney disease focal segmental glomerulosclerosis (FSGS). See also * Actin * Muscle contraction Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle ten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caldesmon
Caldesmon is a protein that in humans is encoded by the ''CALD1'' gene. Caldesmon is a calmodulin binding protein. Like calponin, caldesmon tonically inhibits the ATPase activity of myosin in smooth muscle. This gene encodes a calmodulin- and actin-binding protein that plays an essential role in the regulation of smooth muscle and nonmuscle contraction. The conserved domain of this protein possesses the binding activities to Ca++-calmodulin, actin, tropomyosin, myosin, and phospholipids. This protein is a potent inhibitor of the actin-tropomyosin activated myosin MgATPase, and serves as a mediating factor for Ca++-dependent inhibition of smooth muscle contraction. Alternative splicing of this gene results in multiple transcript variants encoding distinct isoforms. Immunochemistry In diagnostic immunochemistry, caldesmon is a marker for smooth muscle Smooth muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations (''ban ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine. Phospholipids are a key component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. In eukaryotes, cell membranes also contain another class of lipid, sterol, interspersed among the phospholipids. The combination provides fluidity in two dimensions combined with mechanical strength against rupture. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science. The first phospholipid identified in 1847 as such in biological tissues was lecith ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases. Structure Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 kDa). The protein has two approximately symmetrical globular domains (the N- and C- domains) each containing a pair of EF hand motifs separated by a flexible linker region for a total of four Ca2+ binding sites, two in each globular domain. In the Ca2+-free state, the helices that form the four EF-hands are collapsed in a compact orientation, and the central linker is disordered; in the Ca2+-saturated state, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S100 Protein
The S100 proteins are a family of low molecular-weight proteins found in vertebrates characterized by two calcium-binding sites that have helix-loop-helix ("EF-hand-type") conformation. At least 21 different S100 proteins are known. They are encoded by a family of genes whose symbols use the ''S100'' prefix, for example, ''S100A1'', ''S100A2'', ''S100A3''. They are also considered as damage-associated molecular pattern molecules (DAMPs), and knockdown of aryl hydrocarbon receptor downregulates the expression of S100 proteins in THP-1 cells. Structure Most S100 proteins consist of two identical polypeptides (homodimeric), which are held together by noncovalent bonds. They are structurally similar to calmodulin. They differ from calmodulin, though, on the other features. For instance, their expression pattern is cell-specific, i.e. they are expressed in particular cell types. Their expression depends on environmental factors. In contrast, calmodulin is a ubiquitous and universa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |