|
CNN1
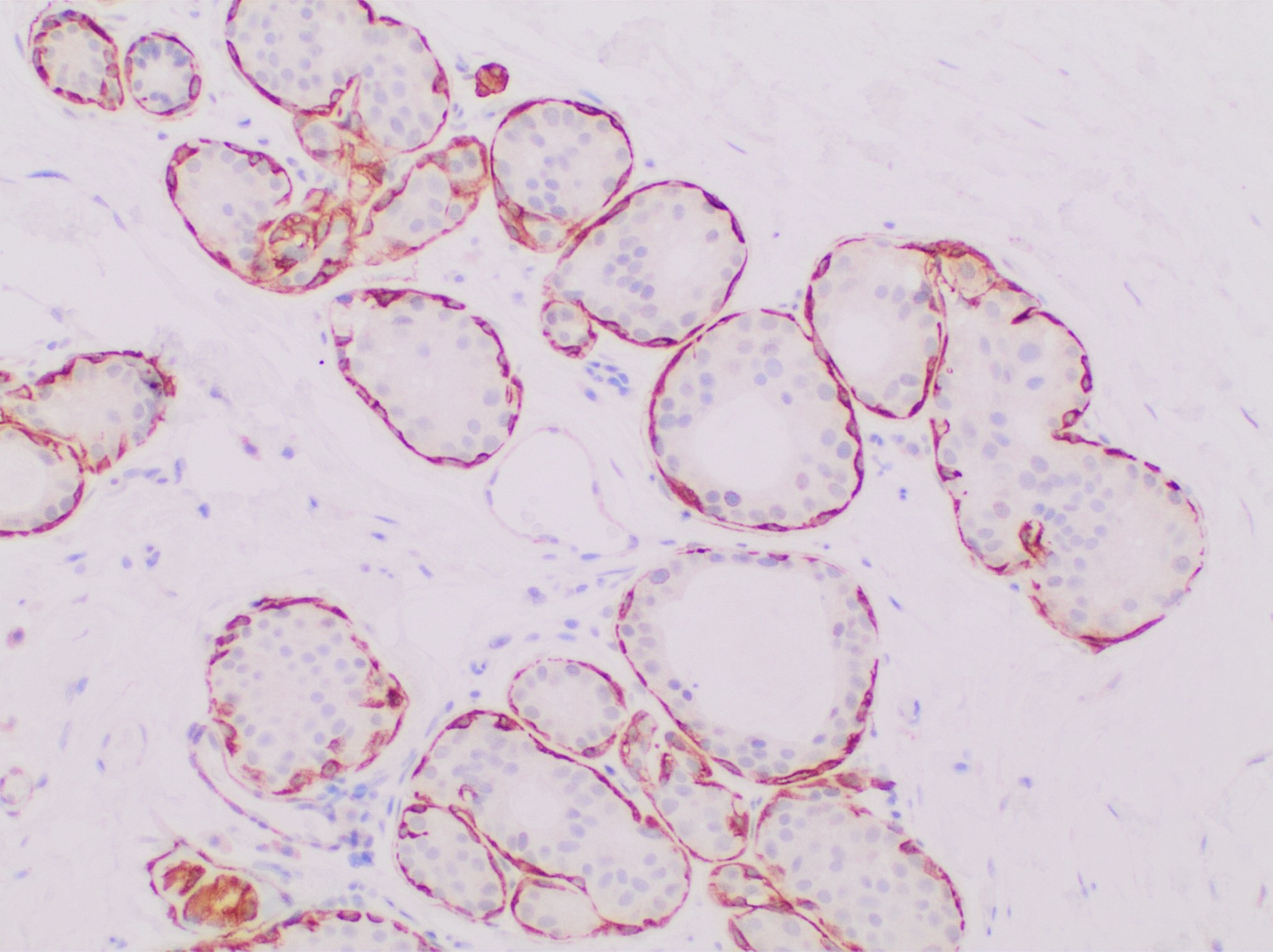
Calponin 1 is a basic smooth muscle protein that in humans is encoded by the ''CNN1'' gene. The ''CNN1'' gene is located at 19p13.2-p13.1 in the human chromosomal genome and contains 7 exons, encoding the protein calponin 1, an actin filament-associated regulatory protein. Human calponin 1 is a 33.2-KDa protein consists of 297 amino acids with an isoelectric point of 9.1, thus calponin 1 is also known as basic calponin. Evolution Three homologous genes, ''Cnn1'', ''Cnn2'' and ''Cnn3'', have evolved in vertebrates, encoding three isoforms of calponin: calponin 1, calponin 2, calponin 3, respectively. Protein sequence alignment shows that calponin 1 is highly conserved in mammals but more diverged among lower vertebrates. Smooth muscle-specific expression The expression of CNN1 is specific to differentiated mature smooth muscle cells, suggesting a role in contractile functions. Calponin 1 is up-regulated in smooth muscle tissues during postnatal development with a higher conte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNN1 Wikipage
Calponin 1 is a basic smooth muscle protein that in humans is encoded by the ''CNN1'' gene. The ''CNN1'' gene is located at 19p13.2-p13.1 in the human chromosomal genome and contains 7 exons, encoding the protein calponin 1, an actin filament-associated regulatory protein. Human calponin 1 is a 33.2-KDa protein consists of 297 amino acids with an isoelectric point of 9.1, thus calponin 1 is also known as basic calponin. Evolution Three homologous genes, ''Cnn1'', ''Cnn2'' and ''Cnn3'', have evolved in vertebrates, encoding three isoforms of calponin: calponin 1, calponin 2, calponin 3, respectively. Protein sequence alignment shows that calponin 1 is highly conserved in mammals but more diverged among lower vertebrates. Smooth muscle-specific expression The expression of CNN1 is specific to differentiated mature smooth muscle cells, suggesting a role in contractile functions. Calponin 1 is up-regulated in smooth muscle tissues during postnatal development with a higher content ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin 3, Acidic
Calponin 3. acidic is a protein that in humans is encoded by the ''CNN3'' gene. The ''CNN3'' gene is located at 1p22-p21 in the human chromosomal genome. ''CNN3'' gene contains 7 exons and encodes calponin 3, a 36.4-kDa protein consisting of 329 amino acids with isoelectric point (pI) of 5.84. Calponin 3 is known as acidic calponin. Among three isoforms of calponin, less is known for the gene regulation and function of calponin 3. Nonetheless, much has been learned from extensive studies on the homologous genes ''CNN1'' and ''CNN2'' that encode calponin 1 and calponin 2. Evolution ''CNN3'' is one of the three homologous calponin isoform genes. Calponin 3 is significantly diverged from calponin 1 and calponin 2 in the C terminal variable region. The higher degree of divergence among vertebrate ''CNN3'' genes than that in the ''CNN1'' and ''CNN2'' gene families suggests possibly earlier emergence of ''CNN3'', indicating that calponin 3 may represent a prototype of calponin ances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin Structure Wikigene Final
Calponin is a calcium binding protein. Calponin tonically inhibits the ATPase activity of myosin in smooth muscle. Phosphorylation of calponin by a protein kinase, which is dependent upon calcium binding to calmodulin, releases the calponin's inhibition of the smooth muscle ATPase. Structure and function Calponin is mainly made up of α-helices with hydrogen bond turns. It is a binding protein and is made up of three domains. These domains in order of appearance are Calponin Homology (CH), regulatory domain (RD), and Click-23, domain that contains the calponin repeats. At the CH domain calponin binds to α-actin and filamin and binds to actin within the RD domain. Calmodulin, when activated by calcium may bind weakly to the CH domain and inhibit calponin binding with α-actin. Calponin is responsible for binding many actin binding proteins, phospholipids, and regulates the actin/myosin interaction. Calponin is also thought to negatively affect the bone making process due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin Homology Domain
Calponin homology domain (or CH domain) is a family of actin binding domains found in both cytoskeletal proteins and signal transduction proteins. The domain is about 100 amino acids in length and is composed of four alpha helices. It comprises the following groups of actin-binding domains: * Actinin-type (including spectrin, fimbrin, ABP-280) * Calponin-type A comprehensive review of proteins containing this type of actin-binding domains is given in. The CH domain is involved in actin binding in some members of the family. However, in calponins there is evidence that the CH domain is not involved in its actin binding activity. Most proteins have two copies of the CH domain, however some proteins such as calponin and the human vav proto-oncogene () have only a single copy. The structure of an example CH domain has been determined using X-ray crystallography. Examples Human genes encoding calponin homology domain-containing proteins include: * ACTN1, ACTN2, ACTN3, ACTN4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calponin
Calponin is a calcium binding protein. Calponin tonically inhibits the ATPase activity of myosin in smooth muscle. Phosphorylation of calponin by a protein kinase, which is dependent upon calcium binding to calmodulin, releases the calponin's inhibition of the smooth muscle ATPase. Structure and function Calponin is mainly made up of α-helices with hydrogen bond turns. It is a binding protein and is made up of three domains. These domains in order of appearance are Calponin Homology (CH), regulatory domain (RD), and Click-23, domain that contains the calponin repeats. At the CH domain calponin binds to α-actin and filamin and binds to actin within the RD domain. Calmodulin Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the bind ..., when activated by calcium may bind weakly to the C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinin Alpha 1
Alpha-actinin-1 is a protein that in humans is encoded by the ''ACTN1'' gene. Function Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of cytoskeletal proteins, including the alpha and beta spectrins and dystrophins. Alpha-actinin-1 is an F-actin cross-linking protein – a bundling protein that is thought to anchor actin to a number of intracellular structures. Alpha-actinin-1 is a non-muscle cytoskeletal isoform found along microfilament bundles and adherens-type junctions, where it is involved in binding actin to the membrane. In contrast, skeletal, cardiac, and smooth muscle isoforms are localized to the Z-disc and analogous dense bodies, where they help anchor the myofibrillar actin filaments. Interactions Alpha-actinin-1 has been shown to interact with: * CDK5R1, * CDK5R2, * Collagen, type XVII, alpha 1, * GIPC1, * PDLIM1, * Protein kinase N1, * SSX2IP, and * Zyxin. *PTPRT (PTPrho) See also * Actinin Actinin is a microfilamen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
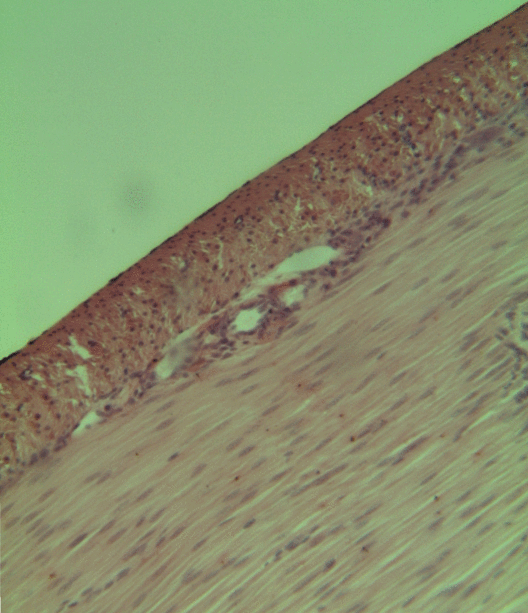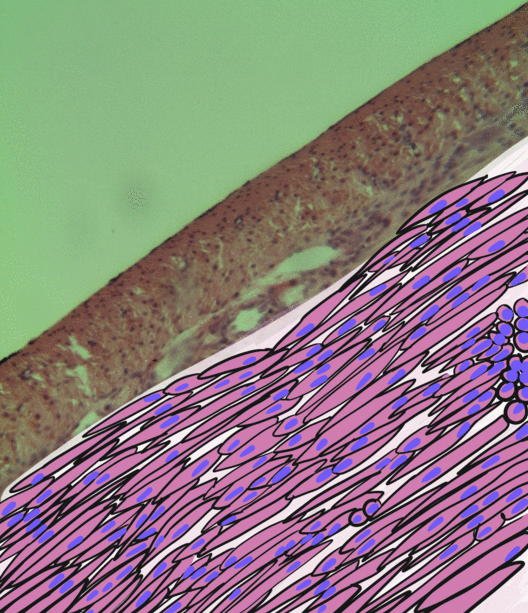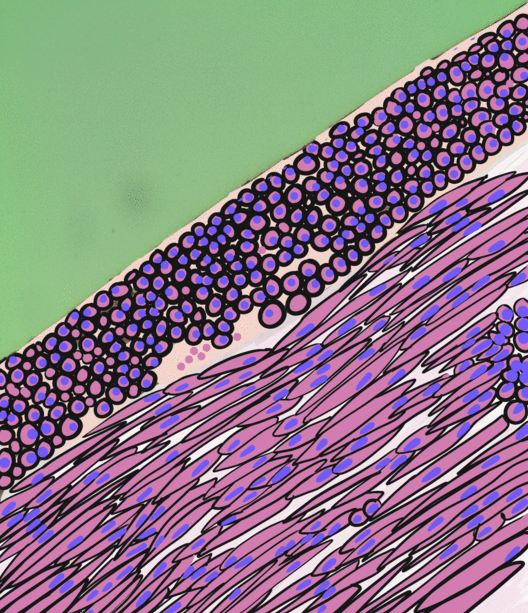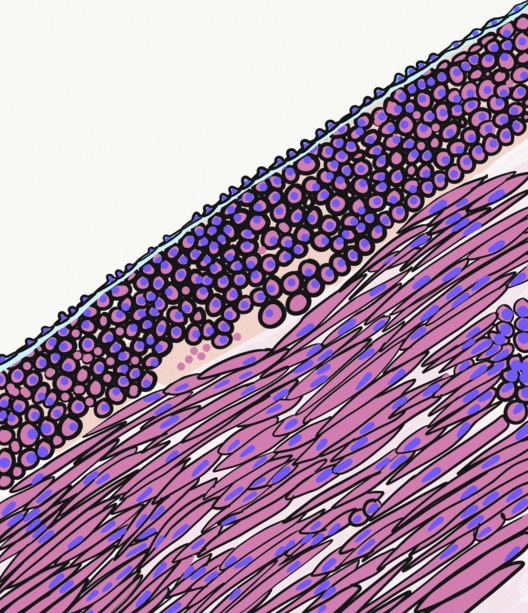
Smooth Muscle Tissue
Smooth muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium. Smooth muscle is found in the walls of hollow organs, including the stomach, intestines, bladder and uterus; in the walls of passageways, such as blood, and lymph vessels, and in the tracts of the respiratory, urinary, and reproductive systems. In the eyes, the ciliary muscles, a type of smooth muscle, dilate and contract the iris and alter the shape of the lens. In the skin, smooth muscle cells such as those of the arrector pili cause hair to stand erect in response to cold temperature or fear. Structure Gross anatomy Smooth muscle is grouped into two types: single-unit smooth muscle, also known as visceral smooth muscle, and multiunit smooth muscle. M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrin
Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filamin
Filamins are a class of proteins that hold two actin filaments at large angles. Filamin protein in mammals is made up of an actin-binding domain at its N-terminus that is followed by 24 immunoglobulin-like repeat modules of roughly 95 amino acids. There are two hinge regions; between repeats 15-16 and 23-24. Filamin gets cleaved at these hinge regions to generate smaller fragments of the protein. Filamin has two actin-binding sites with a V-linkage between them, so that it cross-links actin filaments into a network with the filaments orientated almost at right angles to one another. Filamin proteins include: * FLNA * FLNB * FLNC Over-expression of FLNA stops the regeneration of bladder carcinoma (BC) cells, by inhibiting the cell cycle and inducing apoptosis of BC cells. FLNA has also been shown to reduce the mobility Mobility may refer to: Social sciences and humanities * Economic mobility, ability of individuals or families to improve their economic status * Geographic mobi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |