|
CLEC10A
C-type lectin domain family 10 member A (CLEC10A) also designated as CD301 is a protein that in humans is encoded by the ''CLEC10A'' gene. CLEC10A is part of the C-type lectin superfamily and binds to ''N''-Acetylgalactosamine (GalNAc). It is mainly expressed on myeloid cells and also on oocytes and very early stages of embryogenesis. CLEC10A is used as a marker of the CD1c+ dendritic cell subgroup, also called cDC2. The actions of CLEC10A are diverse, depending on the ligand and environment. Function Generally, C-type lectins bind carbohydrate moieties usually in the presence of Ca2+ and have diverse functions, such as cell adhesion, cell-cell signalling, glycoprotein turnover, and roles in inflammation and immune response. CLEC10A is a type II transmembrane protein (passing one time through the membrane and oriented with the N terminus inward) that induces endocytosis after ligand binding. To release the ligand in the endosome, participating Ca2+ ions have to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * CD302 (C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose
Galactose (, '' galacto-'' + '' -ose'', "milk sugar"), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule. Galactan is a polymeric form of galactose found in hemicellulose, and forming the core of the galactans, a class of natural polymeric carbohydrates. D-Galactose is also known as brain sugar since it is a component of glycoproteins (oligosaccharide-protein compounds) found in Nerve tissue, nerve tissue. Etymology The word ''galactose'' was coined by Charles Weissman in the mid-19th century and is derived from Greek ''galaktos'' (of milk) and the generic chemical suffix for sugars ''-ose''. The etymology is comparable to that of the word '' lactose'' in that both contain roots meaning "milk sugar". Lactose is a disaccharide of galactose plus glucose. Structure and isomerism Gal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 12
Interleukin 12 (IL-12) is an interleukin that is naturally produced by dendritic cells, macrophages, neutrophils, and human B- lymphoblastoid cells ( NC-37) in response to antigenic stimulation. IL-12 belongs to the family of interleukin-12. IL-12 family is unique in comprising the only heterodimeric cytokines, which includes IL-12, IL-23, IL-27 and IL-35. Despite sharing many structural features and molecular partners, they mediate surprisingly diverse functional effects. Gene and structure IL12 is a heterodimeric cytokine encoded by two separate genes, IL-12A (p35) and IL-12B (p40). The active heterodimer (referred to as 'p70'), and a homodimer of p40 are formed following protein synthesis. IL12A is composed of a bundle of four alpha helices. IL12B has three beta sheet domains. Functions IL-12 is involved in the differentiation of naive T cells into Th1 cells. It is known as a T cell-stimulating factor, which can stimulate the growth and function of T cells. It stimulate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 10
Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti- inflammatory cytokine. In humans, interleukin 10 is encoded by the ''IL10'' gene. IL-10 signals through a receptor complex consisting of two IL-10 receptor-1 and two IL-10 receptor-2 proteins. Consequently, the functional receptor consists of four IL-10 receptor molecules. IL-10 binding induces STAT3 signalling via the phosphorylation of the cytoplasmic tails of IL-10 receptor 1 + IL-10 receptor 2 by JAK1 and Tyk2 respectively. Gene and protein structure The IL-10 protein is a homodimer; each of its subunits is 178-amino-acid long. IL-10 is classified as a class-2 cytokine, a set of cytokines including IL-19, IL-20, IL-22, IL-24 (Mda-7), IL-26 and interferons type-I (IFN-alpha, -beta, -epsilon, -kappa, -omega), type-II (IFN-gamma) and type-III (IFN-lambda, also known as IL-28A, IL-28B, and IL-29). Expression and synthesis In humans, IL-10 is encoded by the ''IL10'' gene, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunosuppression
Immunosuppression is a reduction of the activation or efficacy of the immune system. Some portions of the immune system itself have immunosuppressive effects on other parts of the immune system, and immunosuppression may occur as an adverse reaction to treatment of other conditions. In general, deliberately induced immunosuppression is performed to prevent the body from rejecting an organ transplant. Additionally, it is used for treating graft-versus-host disease after a bone marrow transplant, or for the treatment of auto-immune diseases such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome, or Crohn's disease. This is typically done using medications, but may involve surgery (splenectomy), plasmapheresis, or radiation. A person who is undergoing immunosuppression, or whose immune system is weak for some other reasons (such as chemotherapy or HIV), is said to be ''immunocompromised''. Deliberately induced Administration of immunosuppressive medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T Cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface. T cells are born from hematopoietic stem cells, found in the bone marrow. Developing T cells then migrate to the thymus gland to develop (or mature). T cells derive their name from the thymus. After migration to the thymus, the precursor cells mature into several distinct types of T cells. T cell differentiation also continues after they have left the thymus. Groups of specific, differentiated T cell subtypes have a variety of important functions in controlling and shaping the immune response. One of these functions is immune-mediated cell death, and it is carried out by two major subtypes: CD8+ "killer" and CD4+ "helper" T cells. (These are named for the presence of the cell surface proteins CD8 or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
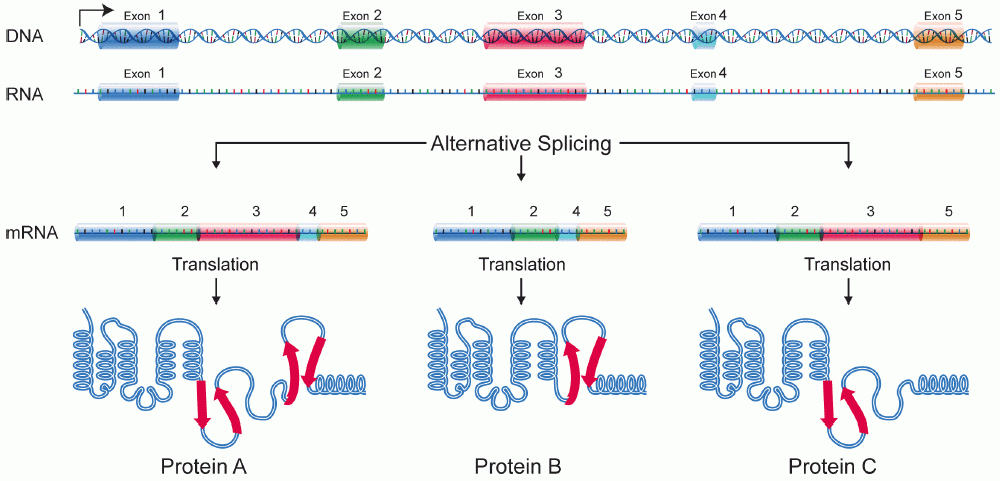
Protein Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments ( exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions genes revealed by the human genome project and the large diversity of proteins seen in an organism: different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exon
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term ''exon'' refers to both the DNA sequence within a gene and to the corresponding sequence in RNA transcripts. In RNA splicing, introns are removed and exons are covalently joined to one another as part of generating the mature RNA. Just as the entire set of genes for a species constitutes the genome, the entire set of exons constitutes the exome. History The term ''exon'' derives from the expressed region and was coined by American biochemist Walter Gilbert in 1978: "The notion of the cistron… must be replaced by that of a transcription unit containing regions which will be lost from the mature messengerwhich I suggest we call introns (for intragenic regions)alternating with regions which will be expressedexons." This definition was originally made for protein-coding transcripts that are spliced before being translated. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTPRC
Protein tyrosine phosphatase, receptor type, C also known as PTPRC is an enzyme that, in humans, is encoded by the ''PTPRC'' gene. PTPRC is also known as CD45 antigen (CD stands for cluster of differentiation), which was originally called leukocyte common antigen (LCA). Function The protein product of this gene, best known as CD45, is a member of the protein tyrosine phosphatase (PTP) family. PTPs are signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. CD45 contains an extracellular domain, a single transmembrane segment, and two tandem intracytoplasmic catalytic domains, and thus belongs to the receptor type PTP family. CD45 is a type I transmembrane protein that is present in various isoforms on all differentiated hematopoietic cells (except erythrocytes and plasma cells). CD45 has been shown to be an essential regulator of T- and B-cell antigen receptor signaling. It function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also influence adaptive immune responses and exert tissue repair functions. There are at least three subclasses of monocytes in human blood based on their phenotypic receptors. Structure Monocytes are amoeboid in appearance, and have nongranulated cytoplasm. Thus they are classified as agranulocytes, although they might occasionally display some azurophil granules and/or vacuoles. With a diameter of 15–22 μm, monocytes are the largest cell type in peripheral blood. Monocytes are mononuclear cells and the ellipsoidal nucleus is often lobulated/indented, causing a bean-shaped or kidney-shaped appearance. Monocytes compose 2% to 10% of all leukocytes in the human body. Development Monocytes are produced by the bone marrow from precursors ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.png)