|
Thermodynamic Systems
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics. Thermodynamic systems can be passive and active according to internal processes. According to internal processes, passive systems and active systems are distinguished: passive, in which there is a redistribution of available energy, active, in which one type of energy is converted into another. Depending on its interaction with the environment, a thermodynamic system may be an isolated system, a Closed system#In thermodynamics, closed system, or an Open system (systems theory), open system. An isolated system does not exchange matter or energy with its surroundings. A closed system may exchange heat, experience forces, and exert forces, but does not exchange matter. An open system can interact with its surroundings by exchanging both matter and energy. The physical condition of a thermodynamic system at a given time is described by its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diagram Systems
A diagram is a symbolic Depiction, representation of information using Visualization (graphics), visualization techniques. Diagrams have been used since prehistoric times on Cave painting, walls of caves, but became more prevalent during the Age of Enlightenment, Enlightenment. Sometimes, the technique uses a Three-dimensional space, three-dimensional visualization which is then graphical projection, projected onto a two-dimensional surface. The word ''graphics, graph'' is sometimes used as a synonym for diagram. Overview The term "diagram" in its commonly used sense can have a general or specific meaning: * ''visual information device'' : Like the term "illustration", "diagram" is used as a collective term standing for the whole class of technical genres, including graphics, graphs, technical drawings and tables. * ''specific kind of visual display'' : This is the genre that shows qualitative data with shapes that are connected by lines, arrows, or other visual links. In scie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is a different material, in its own separate phase. (See .) More precisely, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter (as where oil and water separate into distinct phases, both in the liquid state). Types of phases Distinct phases may be described as different states of matter such as gas, liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirk Ter Haar
Dirk ter Haar FRSE FIP DSc (; 19 April 1919 –3 September 2002) was an Anglo-Dutch physicist. He was emeritus fellow of University of Oxford. Life Dirk ter Haar was born at Oosterwolde in Friesland in the north of the Netherlands on 19 April 1919. He studied physics as an undergraduate at the Leiden University. In 1946 he was a research fellow of Niels Bohr at the Institute for Theoretical Physics in Copenhagen (now the Niels Bohr Institute), and returned to Leiden in 1948 to obtain his PhD. His supervisor was the renowned Hendrik Kramers and his PhD dissertation was on the origin of the Solar System. From 1947 to 1950 he was a visiting associate professor of physics at Purdue University. In 1950 he obtained a post as professor of physics at the University of St. Andrews, and later became a British citizen. In 1952 he was elected a Fellow of the Royal Society of Edinburgh. His proposers were Jack Allen, David Jack, Daniel Edwin Rutherford and Edward Thomas Copson. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert A
The name Robert is an ancient Germanic given name, from Proto-Germanic "fame" and "bright" (''Hrōþiberhtaz''). Compare Old Dutch ''Robrecht'' and Old High German ''Hrodebert'' (a compound of ''Hrōþ, Hruod'' () "fame, glory, honour, praise, renown, godlike" and ''berht'' "bright, light, shining"). It is the second most frequently used given name of ancient Germanic origin.Reaney & Wilson, 1997. ''Dictionary of English Surnames''. Oxford University Press. It is also in use Robert (surname), as a surname. Another commonly used form of the name is Rupert (name), Rupert. After becoming widely used in Continental Europe, the name entered England in its Old French form ''Robert'', where an Old English cognate form (''Hrēodbēorht'', ''Hrodberht'', ''Hrēodbēorð'', ''Hrœdbœrð'', ''Hrœdberð'', ''Hrōðberχtŕ'') had existed before the Norman Conquest. The feminine version is Roberta (given name), Roberta. The Italian, Portuguese, and Spanish form is Roberto (given name), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edward A
Edward is an English male name. It is derived from the Anglo-Saxon name ''Ēadweard'', composed of the elements '' ēad'' "wealth, fortunate; prosperous" and '' weard'' "guardian, protector”. History The name Edward was very popular in Anglo-Saxon England, but the rule of the Norman and Plantagenet dynasties had effectively ended its use amongst the upper classes. The popularity of the name was revived when Henry III named his firstborn son, the future Edward I, as part of his efforts to promote a cult around Edward the Confessor, for whom Henry had a deep admiration. Variant forms The name has been adopted in the Iberian peninsula since the 15th century, due to Edward, King of Portugal, whose mother was English. The Spanish/Portuguese forms of the name are Eduardo and Duarte. Other variant forms include French Édouard, Italian Edoardo and Odoardo, German, Dutch, Czech and Romanian Eduard and Scandinavian Edvard. Short forms include Ed, Eddy, Eddie, Ted, Teddy a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ilya Prigogine
Viscount Ilya Romanovich Prigogine (; ; 28 May 2003) was a Belgian physical chemist of Russian-Jewish origin, noted for his work on dissipative structures, complex systems, and irreversibility. Prigogine's work most notably earned him the 1977 Nobel Prize in Chemistry “for his contributions to non-equilibrium thermodynamics, particularly the theory of dissipative structures”, as well as the Francqui Prize in 1955, and the Rumford Medal in 1976. Biography Early life and studies Prigogine was born in Moscow a few months before the October Revolution of 1917, into a Jewish family. His father, Ruvim (Roman) Abramovich Prigogine, was a chemist who studied at the Imperial Moscow Technical School and owned a soap factory; his mother, Yulia Vikhman, was a pianist who attended the Moscow Conservatory. In 1921, the factory having been nationalized by the new Soviet regime and the feeling of insecurity rising amidst the civil war, the family left Russia. After a brief period i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Closed System
A closed system is a natural physical system that does not allow transfer of matter in or out of the system, althoughin the contexts of physics, chemistry, engineering, etc.the transfer of energy (e.g. as work or heat) is allowed. Physics In classical mechanics In nonrelativistic classical mechanics, a closed system is a physical system that does not exchange any matter with its surroundings, and is not subject to any net force whose source is external to the system. A closed system in classical mechanics would be equivalent to an isolated system in thermodynamics. Closed systems are often used to limit the factors that can affect the results of a specific problem or experiment. In thermodynamics In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings. An isolated system cannot exchange any heat, work, or matter with the surroundings, while an open system can exchange energy and matter. (This scheme of definition of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
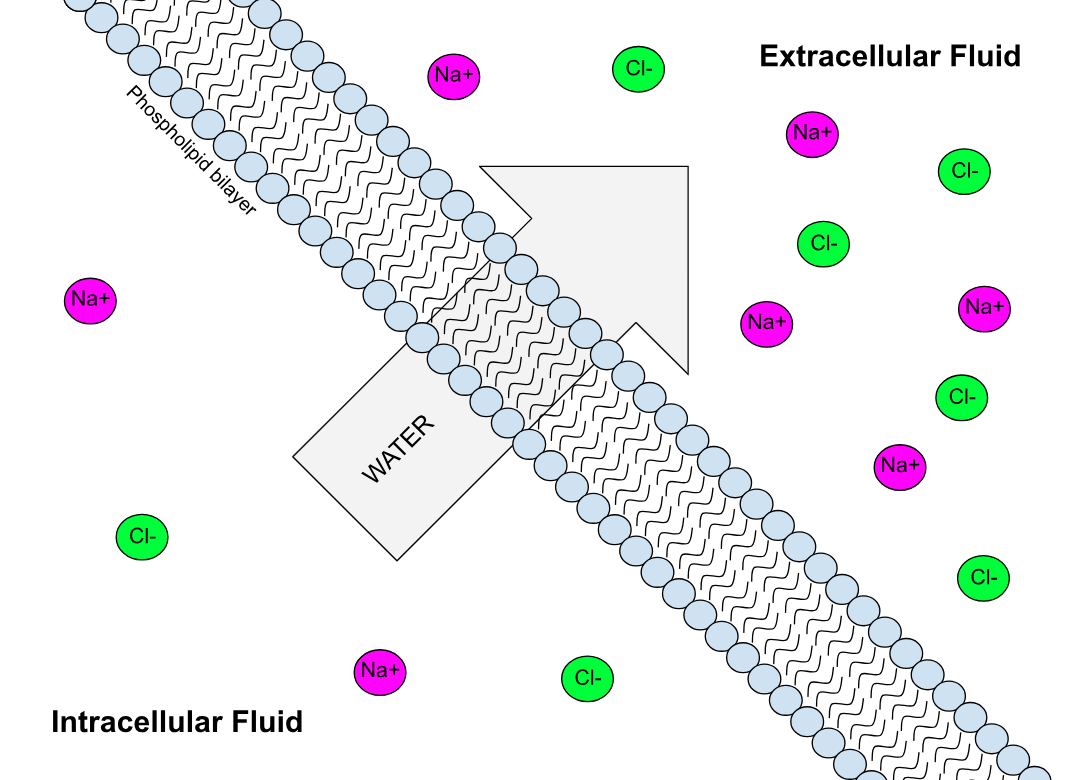
Semipermeable Membrane
Semipermeable membrane is a type of synthetic or biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes Phospholipid bilayer A phospholipid bilayer is an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diathermal Wall
In thermodynamics, a diathermal wall between two thermodynamic systems allows heat transfer but does not allow transfer of matter across it. The diathermal wall is important because, in thermodynamics, it is customary to assume ''a priori'', for a closed system, the physical existence of transfer of energy across a wall that is impermeable to matter but is not adiabatic, transfer which is called transfer of energy as heat, though it is not customary to label this assumption separately as an axiom or numbered law. Definitions of transfer of heat In theoretical thermodynamics, respected authors vary in their approaches to the definition of quantity of heat transferred. There are two main streams of thinking. One is from a primarily empirical viewpoint (which will here be referred to as the thermodynamic stream), to define heat transfer as occurring only by specified macroscopic mechanisms; loosely speaking, this approach is historically older. The other (which will here be referre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Wall
In thermodynamics, an adiabatic wall between two thermodynamic systems does not allow heat or chemical substances to pass across it, in other words there is no heat transfer or mass transfer. In theoretical investigations, it is sometimes assumed that one of the two systems is the surroundings of the other. Then it is assumed that the work transferred is reversible within the surroundings, but in thermodynamics it is not assumed that the work transferred is reversible within the system. The assumption of reversibility in the surroundings has the consequence that the quantity of work transferred is well defined by macroscopic variables in the surroundings. Accordingly, the surroundings are sometimes said to have a reversible work reservoir. Along with the idea of an adiabatic wall is that of an adiabatic enclosure. It is easily possible that a system has some boundary walls that are adiabatic and others that are not. When some are not adiabatic, then the system is not adiabatically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a number of notable physicists in the 1920s and 1930s. Born shared the 1954 Nobel Prize in Physics with Walther Bothe "for his fundamental research in quantum mechanics, especially in the statistical interpretation of the wave function". Born entered the University of Göttingen in 1904, where he met the three renowned mathematicians Felix Klein, David Hilbert, and Hermann Minkowski. He wrote his PhD thesis on the subject of the stability of elastic wires and tapes, winning the university's Philosophy Faculty Prize. In 1905, he began researching special relativity with Minkowski, and subsequently wrote his habilitation thesis on the Thomson model of the atom. A chance meeting with Fritz Haber in Berlin in 1918 led to discussion of how an io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
System
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its open system (systems theory), environment, is described by its boundaries, structure and purpose and is expressed in its functioning. Systems are the subjects of study of systems theory and other systems sciences. Systems have several common properties and characteristics, including structure, function(s), behavior and interconnectivity. Etymology The term ''system'' comes from the Latin word ''systēma'', in turn from Greek language, Greek ''systēma'': "whole concept made of several parts or members, system", literary "composition"."σύστημα" , Henry George Liddell, Robert Scott, ''A Greek–English Lexicon'', on Pers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |