|
Samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samarium(II) are also known, most notably the monoxide SmO, monochalcogenides SmS, SmSe and SmTe, as well as samarium(II) iodide. The last compound is a common reducing agent in chemical synthesis. Samarium has no significant biological role, and some samarium salts are slightly toxic. Samarium was discovered in 1879 by French chemist Paul-Émile Lecoq de Boisbaudran and named after the mineral samarskite from which it was isolated. The mineral itself was named after a Russian mine official, Colonel Vassili Samarsky-Bykhovets, who thus became the first person to have a chemical element named after him, albeit indirectly. Though classified as a rare-earth element, samarium is the 40th most abundant element in Earth's crust and more common than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium–cobalt Magnet
A samarium–cobalt (SmCo) magnet, a type of rare-earth magnet, is a strong permanent magnet made of two basic elements: samarium and cobalt. They were developed in the early 1960s based on work done by Karl Strnat at Wright-Patterson Air Force Base and Alden Ray at the University of Dayton. In particular, Strnat and Ray developed the first formulation of SmCo5. Samarium–cobalt magnets are generally ranked similarly in strength to neodymium magnets, but have higher temperature ratings and higher coercivity. Attributes Some attributes of samarium-cobalts are: * Samarium-cobalt magnets are extremely resistant to demagnetization. * These magnets have good temperature stability (maximum use temperatures between and ; Curie temperature, Curie temperatures from to . * They are expensive and subject to price fluctuations (cobalt is market price sensitive). * Samarium–cobalt magnets have a strong resistance to corrosion and oxidation resistance, usually do not need to be coat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
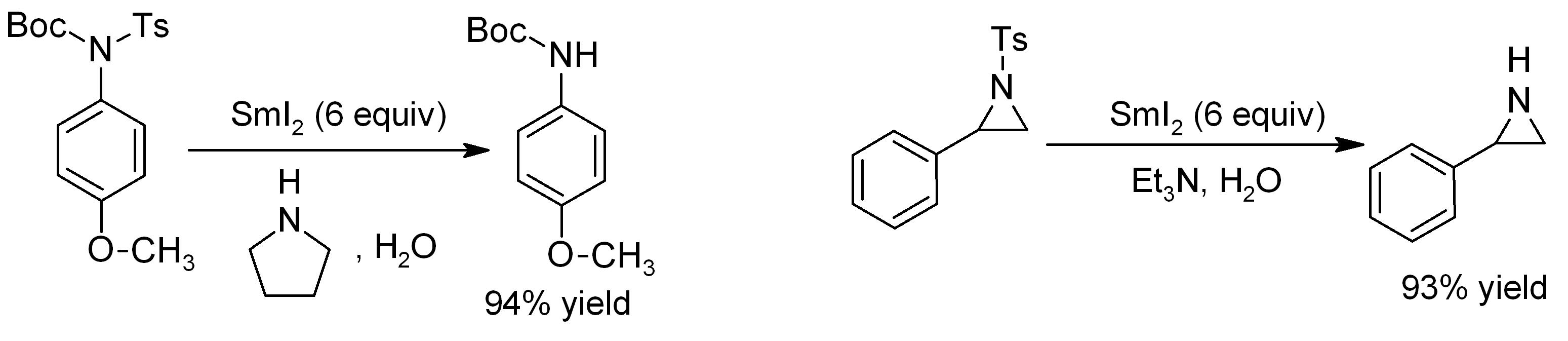
Samarium(II) Iodide
Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis. Structure In samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry. In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands. Preparation Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and either diiodomethane or 1,2-diiodoethane. When prepared in this way, its solutions is most often used without purification of the inorganic reagent. Solid, solvent-free SmI2 forms by high temperature decomposition of samarium(III) iodide (SmI3).G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen Abbau des Samari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium-149
Naturally occurring samarium (62Sm) is composed of five stable isotopes, 144Sm, 149Sm, 150Sm, 152Sm and 154Sm, and two extremely long-lived radioisotopes, 147Sm (half life: 1.06 y) and 148Sm (7 y), with 152Sm being the most abundant (26.75% natural abundance). 146Sm is also fairly long-lived (), but is not long-lived enough to have survived in significant quantities from the formation of the Solar System on Earth, although it remains useful in radiometric dating in the Solar System as an extinct radionuclide. Other than the naturally occurring isotopes, the longest-lived radioisotopes are 151Sm, which has a half-life of 88.8 years, and 145Sm, which has a half-life of 340 days. All of the remaining radioisotopes, which range from 129Sm to 168Sm, have half-lives that are less than two days, and the majority of these have half-lives that are less than 48 seconds. This element also has twelve known isomers with the most stable being 141mSm (t1/2 22.6 minutes), 143m1Sm (t1/2 6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter; it is included due to its chemical similarities with the other 14. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the elements i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium Monochalcogenides
Samarium monochalcogenides are chemical compounds with the composition SmX, where Sm stands for the lanthanide element samarium and X denotes any one of three chalcogen elements, sulfur, selenium or tellurium, resulting in the compounds SmS, SmSe or SmTe. In these compounds, samarium formally exhibits oxidation state +2, whereas it usually assumes the +3 state, resulting in chalcogenides with the chemical formula Sm2X3. Synthesis Single crystals or polycrystals of samarium monochalcogenides can be obtained by reacting the metal with sulfur, selenium or tellurium vapors at high temperature. Thin films can be obtained by magnetron sputtering or electron beam physical vapor deposition, that is bombardment of samarium metal target with electrons in and appropriate gas atmosphere (e.g. hydrogen disulfide for SmS). Properties Samarium monochalcogenides are black semiconducting solids with rock-salt cubic crystal structure. Application of moderate hydrostatic pressure converts them into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul-Émile Lecoq De Boisbaudran
Paul-Émile Lecoq de Boisbaudran, also called François Lecoq de Boisbaudran (18 April 1838 – 28 May 1912), was a French chemist known for his discoveries of the chemical elements gallium, samarium and dysprosium. He developed methods for separation and purification of the rare earth elements and was one of the pioneers of the science of spectroscopy. Biography Lecoq de Boisbaudran was a member of a noble family of Huguenots from the French provinces of Poitou and Angoumois. The Huguenots were French Protestants, a population that was devastated during the French Wars of Religion (1561–1598). The Edict of Nantes (1598) granted substantial civil rights to the Huguenots even though it maintained Catholicism's position as the established religion of France. The Edict of Nantes was overturned by the Edict of Fontainebleau (1685), which officially sanctioned persecution of Protestants. The Lecoq de Boisbaudran family was of considerable fortune until the revocation of the Ed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vassili Samarsky-Bykhovets
Vasili Yevgrafovich Samarsky-Bykhovets (russian: Василий Евграфович Самарский-Быховец; 7 November 1803 – 31 May 1870) was a Russian mining engineer and the chief of Russian Mining Engineering Corps between 1845 and 1861. The mineral samarskite (samarskite-Y, samarskite-Yb and calciosamarskite),Samarskite (in Russian) and are named after him. He was the first person whose name was giv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium (153Sm) Lexidronam
Samarium (153Sm) lexidronam (chemical name Samarium-153-ethylene diamine tetramethylene phosphonate, abbreviated Samarium-153 EDTMP, trade name Quadramet) is a chelated complex of a radioisotope of the element samarium with EDTMP. It is used to treat pain when cancer has spread to the bone. It is injected into a vein and distributed throughout the body, where it is preferentially absorbed in areas where cancer has invaded the bone. The radioisotope 153Sm, with a half-life of 46.3 hours, decays by emitting beta particles (electrons), which kill the nearby cells. Pain begins to improve in the first week for most people and the effects can last several months. It is commonly used in lung cancer, prostate cancer, breast cancer, and osteosarcoma. Side effects Side effects include the following: *Black, tarry stools *Blood in urine/stool *Cough, hoarseness *Fever/chills *Lower back/side pain *Painful or difficult urination *Pinpoint red spots on skin *Irregular heartbeat *Nause ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Control Rod
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing many neutrons without themselves decaying. These elements have different neutron capture cross sections for neutrons of various energies. Boiling water reactors (BWR), pressurized water reactors (PWR), and heavy-water reactors (HWR) operate with thermal neutrons, while breeder reactors operate with fast neutrons. Each reactor design can use different control rod materials based on the energy spectrum of its neutrons. Control rods have been used in nuclear aircraft engines like Project Pluto as a method of control. Operating principle Control rods are inserted into the core of a nuclear reactor and adjusted in order to control the rate of the nuclear chain reaction and, thereby, the thermal power output of the reactor, the rate of stea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium Magnet
A hard_disk_drive.html"_;"title="Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive">Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive_ file:Nd-magnet.jpg.html" ;"title="hard_disk_drive_.html" ;"title="hard_disk_drive.html" ;"title="Nickel-plated neodymium magnet on a bracket from a Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive_">hard_disk_drive.html"_;"title="Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive">Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive_ file:Nd-magnet.jpg">thumb.html" ;"title="hard disk drive">Nickel-plated neodymium magnet on a bracket from a hard disk drive ">hard_disk_drive.html" ;"title="Nickel-plated neodymium magnet on a bracket from a Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive_ file:Nd-magnet.jpg">thumb">Nickel-plated_neodymium_magnet_cubes file:Neodymium_Crystal_Structure_Nd2Fe14B.jpg.html" ;"title="hard disk drive">Nickel-plated neodymiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three carbonate-fluoride minerals, which includes bastnäsite-( Ce) with a formula of (Ce, La)CO3F, bastnäsite-( La) with a formula of (La, Ce)CO3F, and bastnäsite-( Y) with a formula of (Y, Ce)CO3F. Some of the bastnäsites contain OH− instead of F− and receive the name of hydroxylbastnasite. Most bastnäsite is bastnäsite-(Ce), and cerium is by far the most common of the rare earths in this class of minerals. Bastnäsite and the phosphate mineral monazite are the two largest sources of cerium and other rare-earth elements. Bastnäsite was first described by the Swedish chemist Wilhelm Hisinger in 1838. It is named for the Bastnäs mine near Riddarhyttan, Västmanland, Sweden. Bastnäsite also occurs as very high-quality specimens at the Zagi Mountains, Pakistan. Bastnäsite occurs in alkali granite and syenite and in associated pegmatites. It also occurs in carbonatites and in associated fenites and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |