|
Phthalocyanines
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity. It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from , the conjugate base of , are valuable in catalysis, organic solar cells, and photodynamic therapy. Properties Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents. Benzene at 40 °C dissolves less than a milligram of or CuPc per litre. and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are, thermally, very stable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
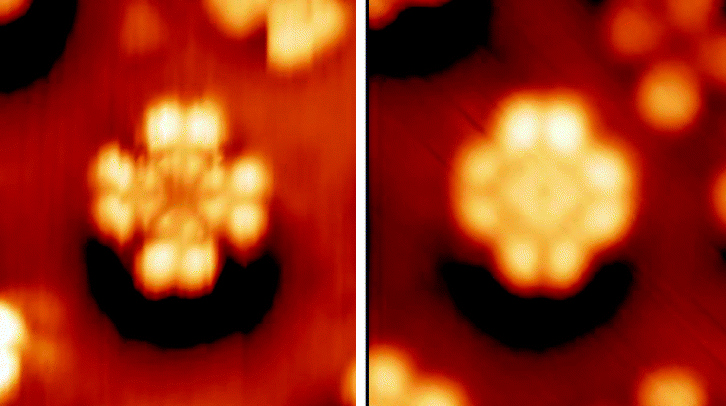
Phthalocyanine STM
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity. It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from , the conjugate base of , are valuable in catalysis, organic solar cells, and photodynamic therapy. Properties Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents. Benzene at 40 °C dissolves less than a milligram of or CuPc per litre. and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are, thermally, very stable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phthalocyanine Blue BN
Copper phthalocyanine (CuPc), also called phthalocyanine blue, phthalo blue and many other names, is a bright, crystalline, synthetic blue pigment from the group of phthalocyanine dyes. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalis and acids. It has the appearance of a blue powder, insoluble in most solvents including water. History The discovery of metal phthalocyanines can be traced to the observation of intensely colored byproducts from reactions of phthalic acid (benzene-1,2-dicarboxylic acid) or its derivatives with sources of nitrogen and metals. CuPc (copper phthalocyanine) was first prepared in 1927 by the reaction of copper(I) cyanide and ''o''-dibromobenzene, which mainly produces colorless phthalonitrile as well as an intensely blue by-product. A couple of years later, workers at Scottish Dyes observed the for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalocyanine
Naphthalocyanine is a cross-shaped organic molecule consisting of 48 carbon, 8 nitrogen and 26 hydrogen atoms, it is a derivative of phthalocyanine. IBM Research labs used it for developing single-molecule logic switches and visualizing charge distribution In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in co ... in a single molecule. Naphthalocyanine derivatives have a potential use in photodynamic cancer treatment. References External links * Timmer, J. (2007''Storing data in molecules: shifting atoms and flipping bits'' ars technica online ccessed 8 September 2007 Phthalocyanines Molecular electronics {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodynamic Therapy
Photodynamic therapy (PDT) is a form of phototherapy involving light and a photosensitizing chemical substance, used in conjunction with molecular oxygen to elicit cell death (phototoxicity). PDT is popularly used in treating acne. It is used clinically to treat a wide range of medical conditions, including wet age-related macular degeneration, psoriasis, atherosclerosis and has shown some efficacy in anti-viral treatments, including herpes. It also treats malignant cancers including head and neck, lung, bladder and particular skin. The technology has also been tested for treatment of prostate cancer, both in a dog model and in human prostate cancer patients. It is recognised as a treatment strategy that is both minimally invasive and minimally toxic. Other light-based and laser therapies such as laser wound healing and rejuvenation, or intense pulsed light hair removal do not require a photosensitizer. Photosensitisers have been employed to sterilise blood plasma and wate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometre
330px, Different lengths as in respect to the molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, -er, American spelling) is a units of measurement, unit of length in the International System of Units (SI), equal to one billionth (short scale) of a metre () and to 1000 picometres. One nanometre can be expressed in scientific notation as , and as metres. History The nanometre was formerly known as the millimicrometre – or, more commonly, the millimicron for short – since it is of a micron (micrometre), and was often denoted by the symbol mμ or (more rarely and confusingly, since it logically should refer to a ''millionth'' of a micron) as μμ. Etymology The name combines the SI prefix ''nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Near Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History The fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |