|
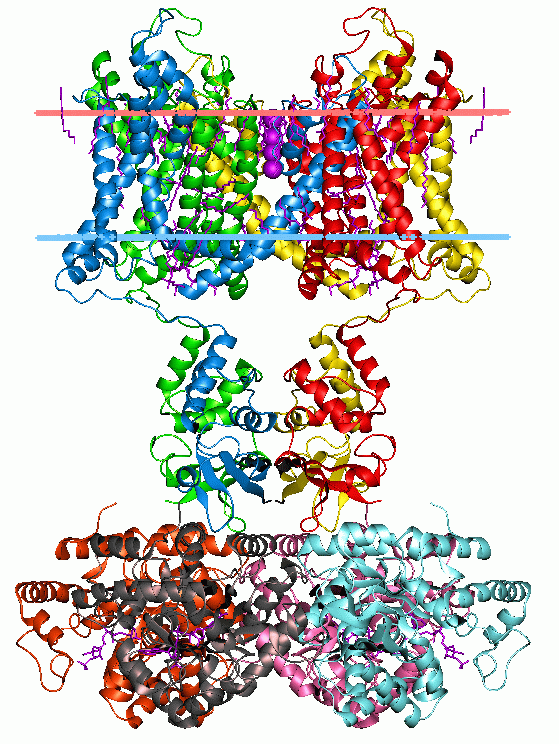
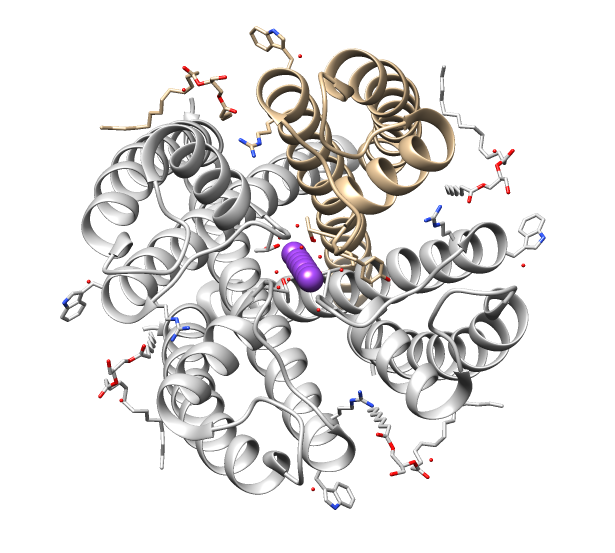
Cys-loop Superfamily
The Cys-loop ligand-gated ion channel superfamily is composed of Nicotinic acetylcholine receptor, nicotinic acetylcholine, GABA A receptor, GABAA, GABAA-rho receptor, GABAA-ρ, Glycine receptor, glycine, 5-HT3 receptor, 5-HT3, and zinc-activated ion channel, zinc-activated (ZAC) Receptor (biochemistry), receptors. These receptors are composed of five protein subunits which form a pentameric arrangement around a central pore. There are usually 2 alpha subunits and 3 other beta, gamma, or delta subunits (some consist of 5 alpha subunits). The name of the family refers to a characteristic loop formed by 13 highly conserved Amino acid, amino acids between two cysteine (Cys) residues, which form a Disulfide, disulfide bond near the N-terminal extracellular domain. Cys-loop receptors are known only in Eukaryote, eukaryotes, but are part of a larger family of pentameric ligand-gated ion channels. Only the Cys-loop clade includes the pair of bridging cysteine residues. The larger superfami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand-gated Ion Channel
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter. When a presynaptic neuron is excited, it releases a neurotransmitter from vesicles into the synaptic cleft. The neurotransmitter then binds to receptors located on the postsynaptic neuron. If these receptors are ligand-gated ion channels, a resulting conformational change opens the ion channels, which leads to a flow of ions across the cell membrane. This, in turn, results in either a depolarization, for an excitatory receptor response, or a hyperpolarization, for an inhibitory response. These receptor proteins are typically composed of at least two different domains: a transmembrane domain which includes the ion pore, and an extracellular domain wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Amino Acids
An aromatic amino acid is an amino acid that includes an aromatic ring. Among the 20 standard amino acids, the following are classically considered aromatic: phenylalanine, tryptophan and tyrosine. Although histidine contains an aromatic ring, its basic properties cause it to be predominantly classified as a polar amino acid. Chemical structure and properties Aromatic amino acids absorb ultraviolet light at a wavelength above 250 nm and produce fluorescence. This characteristic is used in quantitative analysis, notably in determining the concentrations of these amino acids in solution. This achieved through the utilization of a UV spectrophotomer and the Beer-Lambert Law equation. Most proteins will have an absorption maximum at 280 nm due to the presence of aromatic amino acids in their primary structure. However, because several aromatic amino acids exist, this method has low accuracy; in order to mitigate this issue, the desired protein must be pure, and its molar absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Neuroscience
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic. Locating neurotransmitters In molecular biology, communication between neurons typically occurs by chemical transmission across gaps between the cells called synapses. The transmitted chemicals, known as neurotransmitters, regulate a significant fraction of vital body functions. It is possible to anatomically locate neurotransmitters by labeling techniques. It is possible to chemically identify certain neurotransmitters such as catecholamines by fixing neural tissue sectio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionotropic Receptors
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter. When a presynaptic neuron is excited, it releases a neurotransmitter from vesicles into the synaptic cleft. The neurotransmitter then binds to receptors located on the postsynaptic neuron. If these receptors are ligand-gated ion channels, a resulting conformational change opens the ion channels, which leads to a flow of ions across the cell membrane. This, in turn, results in either a depolarization, for an excitatory receptor response, or a hyperpolarization, for an inhibitory response. These receptor proteins are typically composed of at least two different domains: a transmembrane domain which includes the ion pore, and an extracellular domain wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channels
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophysiology
Electrophysiology (from Greek , ''ēlektron'', "amber" etymology of "electron"">Electron#Etymology">etymology of "electron" , ''physis'', "nature, origin"; and , '' -logia'') is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes or electric current or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electric signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electrodiagnosis and monitoring. Definition and scope Classical electrophysiological techniques Principle and mechanisms Electrophysiology is the branch of physiology that pertains broadly to the flow of ions (ion current) in biologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Agonists
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine. Examples include nicotine (by definition), acetylcholine (the endogenous agonist of nAChRs), choline, epibatidine, lobeline, varenicline and cytisine. History Nicotine has been known for centuries for its intoxicating effect. It was first isolated in 1828 from the tobacco plant by German chemists Posselt and Reimann. The discovery of positive effects from nicotine on animal memory was discovered by in vivo researches in the mid 1980s. Those researches led to a new era in studies of nicotinic acetylcholine receptor (nAChR) and their stimulation but until then the focus had mainly been on nicotine addiction. The development of nAChR agonists began in the early 1990s after the discovery of nicotine's positive effects. Some research showed a possible therapy option in preclinical researches. ABT-418 was one of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Microscope
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a higher resolving power than light microscopes and can reveal the structure of smaller objects. A scanning transmission electron microscope has achieved better than 50 pm resolution in annular dark-field imaging mode and magnifications of up to about 10,000,000× whereas most light microscopes are limited by diffraction to about 200 nm resolution and useful magnifications below 2000×. Electron microscopes use shaped magnetic fields to form electron optical lens systems that are analogous to the glass lenses of an optical light microscope. Electron microscopes are used to investigate the ultrastructure of a wide range of biological and inorganic specimens including microorganisms, cells, large molecules, biopsy samples, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Domain
In molecular biology, binding domain is a protein domain which binds to a specific atom or molecule, such as calcium or DNA. A protein domain is a part of a protein sequence and a tertiary structure that can change or evolve, function, and live by itself independent of the rest of the protein chain. Upon binding, proteins may undergo a conformational change. Binding domains are essential for the function of many proteins. They are essential because they help splice, assemble, and translate proteins.Yong, J., T. J. Golembe, D. J. Battle, L. Pellizzoni, and G. Dreyfuss. "SnRNAs Contain Specific SMN-binding Domains That Are Essential for SnRNP Assembly". ''Molecular and Cellular Biology''. U.S. National Library of Medicine, April 2004. Retrieved April 2017. Examples of binding domains include the Zinc finger, which binds to DNA, and EF hand, which binds to calcium. See also *DNA-binding domain *Receptor (biochemistry) In biochemistry and pharmacology, receptors are chemica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Regulation
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. Allosteric regulation is also particularly important in the cell's ability to adjust enzyme activity. The term ''allostery'' comes from the Ancient Greek ''allos'' (), "other", and ''stereos' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |