|
Cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimer (chemistry), dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction. This dimer can be retro-Diels–Alder reaction, restored by heating to give the monomer. The compound is mainly used for the production of cyclopentene and its derivatives. It is popularly used as a precursor to the cyclopentadienyl anion (Cp−), an important ligand in cyclopentadienyl complexes in organometallic chemistry. Production and reactions Cyclopentadiene production is usually not distinguished from dicyclopentadiene since they interconvert. They are obtained from coal tar (about 10–20 g/tonne, t) and by steam Cracking (chemistry), cracking of Petroleum naphtha, naphtha (about 14 kg/t) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula . At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints. The top seven suppliers worldwide together had an annual capacity in 2001 of 179 kilotonnes (395 million pounds). DCPD was discovered in 1885 as a hydrocarbon among the products of pyrolysis of phenol by Henry Roscoe, who didn't identify the structure (that was made during the following decade) but accurately assumed that it was a dimer of some hydrocarbon. History and structure For many years the structure of dicy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of the principal cycloalkenes. History and synthesis Cyclopentene was first prepared by Carl Gärtner in 1893 from iodocyclopentane with potassium hydroxide. He named it pentamethenylene (). Cyclopentene is produced industrially in large amounts by steam cracking of naphtha. In the laboratory, it is prepared by dehydration of cyclopentanol. Substituted cyclopentenes are the product of the vinylcyclopropane-cyclopentene rearrangement. It can also be produced by the catalytic hydrogenation of cyclopentadiene.D. Hönicke, R. Födisch, P. Claus, M. Olson: ''Cyclopentadiene and Cyclopentene'', in: ''Ullmann's Encyclopedia of Industrial Chemistry, Ullmanns Enzyklopädie der Technischen Chemie'' 2002, Wiley-VCH, Weinheim. Reactions The polymeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
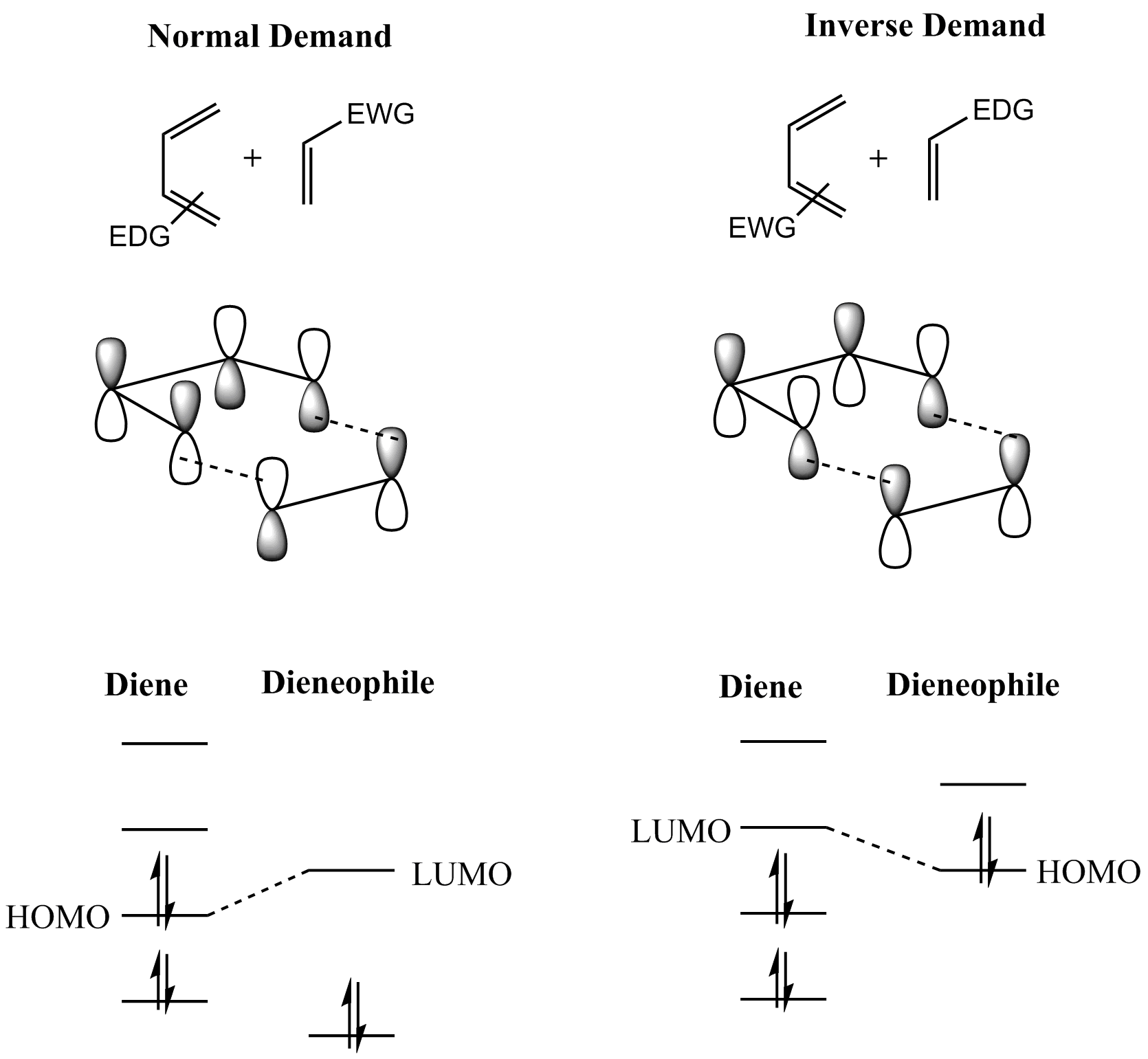
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Anion
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities. Preparation The first salt of cyclopentadienide to be reported was potassium cyclopentadienide, prepared by Johannes Thiele. In 1901 there was not much interest in the topic. Sodium cyclopentadienyl is prepared by treating cyclopentadiene with sodium: : The conversion can be conducted by heating a suspension of molten sodium in dicyclopentadiene.Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878. In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing xylene and rapidly stirring. Sodium hydride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluxional Molecule
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods. Spectroscopic studies Many organometallic compounds exhibit fluxionality. Fluxionality is, however, pervasive. NMR spectroscopy Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates compar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (Metal carbonyl, metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganics, metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AW Cyclopentadiene
A&W, AW, Aw, aW or aw may refer to: Companies * A&W Restaurants ** A&W Root Beer *A&W (Canada) * Addison-Wesley, publishers * Africa World Airlines, IATA code * Prefix for helicopters made by AgustaWestland * Alienware * Allied Waste Industries, Inc, stock symbol on NYSE * Armstrong Whitworth, a British manufacturing company Media and entertainment * ''Accel World'', a Japanese light novel series * ''Active Worlds'', a 3D virtual reality platform * ''Another World'' (TV series), an American soap opera * ''Athletics Weekly'', a monthly track and field magazine published in the United Kingdom * ''Aviation Week'', magazine * Call of Duty: Advanced Warfare, an action video game People * A. H. Weiler (1908 – 2002), ''The New York Times'' film critic whose early reviews were signed with his initials A. W. * A. W. (poet), anonymous 16th century poet * Abraham Washington (A. W.), American professional wrestler and wrestling commentator * Alan Walker (born 1997), English-Nor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the short ton ( United States customary units) and the long ton ( British imperial units). It is equivalent to approximately 2,204.6 pounds, 1.102 short tons, and 0.984 long tons. The official SI unit is the megagram (Mg), a less common way to express the same amount. Symbol and abbreviations The BIPM symbol for the tonne is t, adopted at the same time as the unit in 1879.Table 6 . BIPM. Retrieved on 2011-07-10. Its use is also official for the metric ton in the United States, having been adopted by the United States |
Petroleum Naphtha
Petroleum naphtha is an intermediate hydrocarbon liquid stream derived from the refining of crude oil with CAS-no 64742-48-9. It is most usually desulfurized and then catalytically reformed, which rearranges or restructures the hydrocarbon molecules in the naphtha as well as breaking some of the molecules into smaller molecules to produce a high- octane component of gasoline (or petrol). There are hundreds of different petroleum crude oil sources worldwide and each crude oil has its own unique composition or assay. There are also hundreds of petroleum refineries worldwide and each of them is designed to process either a specific crude oil or specific types of crude oils. Naphtha is a general term as each refinery produces its own naphthas with their own unique initial and final boiling points and other physical and compositional characteristics. Naphthas may also be produced from other material such as coal tar, shale deposits, tar sands, and the destructive distillation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |