|
Cycloheptane
Cycloheptane is a cycloalkane with the molecular formula C7 H14. Cycloheptane is used as a nonpolar solvent for the chemical industry and as an intermediate in the manufacture of chemicals and pharmaceutical drugs. It may be derived by Clemmensen reduction from cycloheptanone. Cycloheptane vapour is irritating to the eyes and may cause respiratory depression if inhaled in large quantity. Conformations Cycloheptane is not a flat molecule, because that would give C-C-C bond angles much greater than the tetrahedral angle of around 109.5°. Instead it is puckered and three-dimensional. One can ask the question of what conformations would have the same angle everywhere (near 109.5°) and all bond lengths equal. If we think of an open chain of seven bonds, there are five dihedral angles that can be chosen, for the sequences (1,2,3,4), (2,3,4,5), and so on. The last bond though should end where the first began, and should form the correct angle with the first bond. This imposes four cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon Solvents
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon dio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkanes
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains are classified as small ( cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formula for cycloalkanes is C''n''H2( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formula for cycloalkanes is C''n''H2(' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Modern On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Producers of cyclohexane account for approximately 11.4% of global demand for benzene. The reaction is highly exothermic, with ΔH(500 K) = -216.37 kJ/mol. Dehydrogenation commenced noticeably above 300 °C, reflecting the favorable entropy for dehydrogenation. : Early Unlike benzene, cyclohexane is not found in natural resources such as coal. For this reason, early investigators synthesized their cyclohexane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctane
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general. Cyclooctane has a camphoraceous odor. Conformations The conformation of cyclooctane has been studied extensively using computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy". The boat-chair conformation (below) is the most stable form. This conformation was confirmed by Allinger and co-workers. The crown conformation (below) is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur. : Synthesis and reactions The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This process affords, among other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
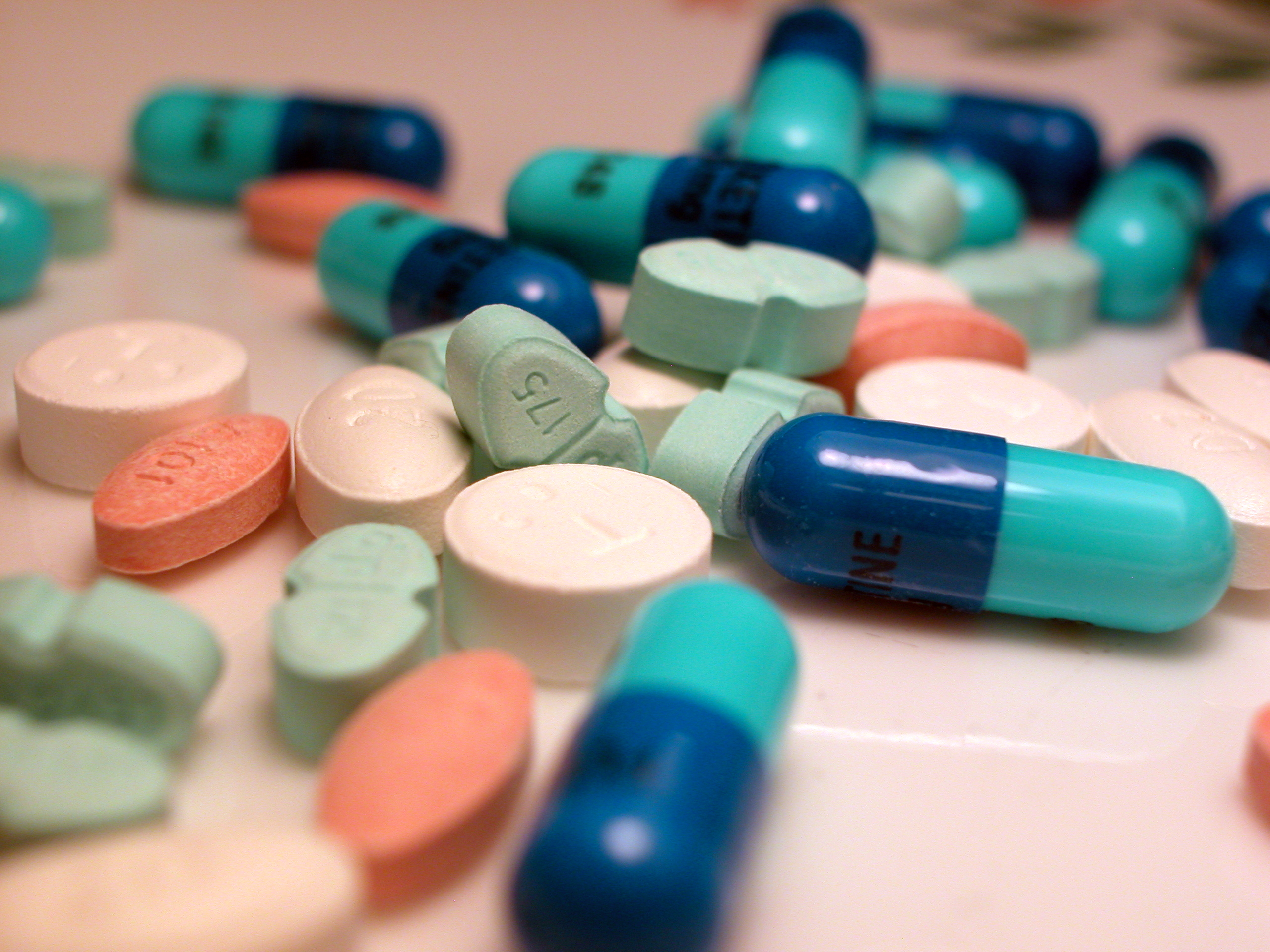
Pharmaceutical Drug
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane Conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds. The internal angles of a regular, flat hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle (the arc cosine of −). Therefore, the cyclohexane ring tends to assume non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. Consider the carbon atoms numbered from 1 to 6 around the ring. If we hold carbon atoms 1, 2, and 3 stationary, with the correct bond lengths and the tetrahedral angle between the two bonds, and then continue by adding carbon atoms 4, 5, and 6 with the correct bond length and the tetrahedral angle, we can var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Improper Rotation
In geometry, an improper rotation,. also called rotation-reflection, rotoreflection, rotary reflection,. or rotoinversion is an isometry in Euclidean space that is a combination of a rotation about an axis and a reflection in a plane perpendicular to that axis. Reflection and inversion are each special case of improper rotation. Any improper rotation is an affine transformation and, in cases that keep the coordinate origin fixed, a linear transformation.. It is used as a symmetry operation in the context of geometric symmetry, molecular symmetry and crystallography, where an object that is unchanged by a combination of rotation and reflection is said to have ''improper rotation symmetry''. Three dimensions In 3 dimensions, improper rotation is equivalently defined as a combination of rotation about an axis and inversion in a point on the axis. For this reason it is also called a rotoinversion or rotary inversion. The two definitions are equivalent because rotation by an angle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chemistry (IUPAC) defines a pseudorotation as a " ereoisomerization resulting in a structure that ''appears'' to have been produced by rotation of the entire initial molecule", the result of which is a "product" that is "superposable on the initial one, unless different positions are distinguished by substitution, including isotopic substitution." Well-known examples are the intramolecular isomerization of trigonal bipyramidal compounds by the Berry pseudorotation mechanism, and the out-of-plane motions of carbon atoms exhibited by cyclopentane, leading to the interconversions it experiences between its many possible conformers (envelope, twist). Note, no angular momentum is generated by this motion. In these and related examples, a small d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihedral Angle
A dihedral angle is the angle between two intersecting planes or half-planes. In chemistry, it is the clockwise angle between half-planes through two sets of three atoms, having two atoms in common. In solid geometry, it is defined as the union of a line and two half-planes that have this line as a common edge. In higher dimensions, a dihedral angle represents the angle between two hyperplanes. The planes of a flying machine are said to be at positive dihedral angle when both starboard and port main planes (commonly called wings) are upwardly inclined to the lateral axis. When downwardly inclined they are said to be at a negative dihedral angle. Mathematical background When the two intersecting planes are described in terms of Cartesian coordinates by the two equations : a_1 x + b_1 y + c_1 z + d_1 = 0 :a_2 x + b_2 y + c_2 z + d_2 = 0 the dihedral angle, \varphi between them is given by: :\cos \varphi = \frac and satisfies 0\le \varphi \le \pi/2. Alternatively, if an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respiratory Depression
Hypoventilation (also known as respiratory depression) occurs when ventilation is inadequate (''hypo'' meaning "below") to perform needed respiratory gas exchange. By definition it causes an increased concentration of carbon dioxide (hypercapnia) and respiratory acidosis. Hypoventilation is not synonymous with respiratory arrest, in which breathing ceases entirely and death occurs within minutes due to hypoxia and leads rapidly into complete anoxia, although both are medical emergencies. Hypoventilation can be considered a precursor to hypoxia and its lethality is attributed to hypoxia with carbon dioxide toxicity. Causes Hypoventilation may be caused by: *A medical condition such as stroke affecting the brainstem *Voluntary breath-holding or underbreathing, for example, hypoventilation training or the Buteyko method. *Medication or drugs, typically when taken in accidental or intentional overdose. Opioids and benzodiazepines in particular are known to cause respiratory depress ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloheptanone
Cycloheptanone, (CH2)6CO, is a cyclic ketone also referred to as suberone. It is a colourless volatile liquid. Cycloheptanone is used as a precursor for the synthesis of pharmaceuticals. Synthesis In 1836, French chemist Jean-Baptiste Boussingault first synthesized cycloheptanone from the calcium salt of dibasic suberic acid. The ketonization of calcium suberate yields calcium carbonate and suberone: :Ca(O2C(CH2)6CO2) → CaCO3 + (CH2)6CO Cycloheptanone is still produced by the cyclization and decarboxylation of suberic acid or suberic acid esters. This reaction is typically conducted in the gas phase at 400–450 °C over alumina doped with zinc oxide or cerium oxide. Cycloheptanone is also produced by the reaction of cyclohexanone with sodium ethoxide and nitromethane. The resulting sodium salt of 1-(nitromethyl)cyclohexanol is added to acetic acid and shaken with hydrogen gas in the presence of W-4 Raney nickel catalyst. Sodium nitrite and acetic acid are then added to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |