|
Cyclo(18)carbon
Cyclooctadeca-1,3,5,7,9,11,13,15,17-nonayne or cyclo 8arbon is an allotrope of carbon with molecular formula . The molecule is a ring of eighteen carbon atoms, connected by alternating triple and single bonds; thus, it is a polyyne and a cyclocarbon. Cyclo 8arbon is the smallest cyclo 'n''arbon predicted to be thermodynamically stable, with a computed strain energy of 72 kilocalories per mole. Above 122K, it explosively decomposes to amorphous graphite. A collaboration of teams at IBM and the University of Oxford team claimed to synthesize it in solid state in 2019 by electrochemical decarbonylation of several sites of a cyclobutanone structure: Later, researchers from Spain have used computational techniques to probe the structural and electronic properties of the molecule, and have discovered it to be an electron acceptor. According to these IBM researchers, the electronic structure of their product consists of alternating triple bonds and single bonds, rather than a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclocarbons
In organic chemistry, a cyclo 'n''arbon (or simply cyclocarbon) is a chemical compound consisting solely of a number ''n'' of carbon atoms covalently linked in a ring. Since the compounds are composed only of carbon atoms, they are allotropes of carbon. Possible bonding patterns include all double bonds (a cyclic cumulene) or alternating single bonds and triple bonds (a cyclic polyyne). As of 2020, the only cyclocarbon that has been synthesized is cyclo 8arbon. Cyclo arbon The (hypothetical) three-carbon member of this family () is also called cyclopropatriene. Cyclo arbon The (hypothetical) six-carbon member of this family () is also called benzotriyne. Cyclo 8arbon The smallest cyclo 'n''arbon predicted to be thermodynamically stable is C18, with a computed strain energy of 72 kilocalories per mole. An IBM/Oxford team claimed to synthesize its molecules in solid state in 2019: According to these IBM researchers, the synthesized cyclocarbon has alternating triple a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclocarbon
In organic chemistry, a cyclo 'n''arbon (or simply cyclocarbon) is a chemical compound consisting solely of a number ''n'' of carbon atoms covalently linked in a ring. Since the compounds are composed only of carbon atoms, they are allotropes of carbon. Possible bonding patterns include all double bonds (a cyclic cumulene) or alternating single bonds and triple bonds (a cyclic polyyne). As of 2020, the only cyclocarbon that has been synthesized is cyclo 8arbon. Cyclo arbon The (hypothetical) three-carbon member of this family () is also called cyclopropatriene. Cyclo arbon The (hypothetical) six-carbon member of this family () is also called benzotriyne. Cyclo 8arbon The smallest cyclo 'n''arbon predicted to be thermodynamically stable is C18, with a computed strain energy of 72 kilocalories per mole. An IBM/Oxford team claimed to synthesize its molecules in solid state in 2019: According to these IBM researchers, the synthesized cyclocarbon has alternating triple a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, Carbon nanobud, nanobuds and Graphene nanoribbon, nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Diamond Diamond is a well-known allotrope of carbon. The hardness, extremely high refractive index, and high Dispersion (optics), dispersion of light make diamond useful for industrial applications and for jewelry. Diamond is the hardest known natural min ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthese C18
''Synthese'' () is a scholarly periodical specializing in papers in epistemology, methodology, and philosophy of science, and related issues. Its subject area is divided into four specialties, with a focus on the first three: (1) "epistemology, methodology, and philosophy of science, all broadly understood"; (2) "foundations of logic and mathematics, where 'logic', 'mathematics', and 'foundations' are all broadly understood"; (3) "formal methods in philosophy, including methods connecting philosophy to other academic fields"; and (4) "issues in ethics and the history and sociology of logic, mathematics, and science that contribute to the contemporary studies". As of 2022, according to Google Scholar's metrics ( h-5 index and h-5 index median), it is the top philosophy journal, but other metrics do not rank the journal as highly. Overview Published articles include specific treatment of methodological issues in science such as induction, probability, causation, statistics, symboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group IV Semiconductors
A group is a number of persons or things that are located, gathered, or classed together. Groups of people * Cultural group, a group whose members share the same cultural identity * Ethnic group, a group whose members share the same ethnic identity * Religious group (other), a group whose members share the same religious identity * Social group, a group whose members share the same social identity * Tribal group, a group whose members share the same tribal identity * Organization, an entity that has a collective goal and is linked to an external environment * Peer group, an entity of three or more people with similar age, ability, experience, and interest Social science * In-group and out-group * Primary, secondary, and reference groups * Social group * Collectives Science and technology Mathematics * Group (mathematics), a set together with a binary operation satisfying certain algebraic conditions Chemistry * Functional group, a group of atoms which provide s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Compounds
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glass. Its electrical resistivity and conductivity, resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities ("doping (semiconductor), doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
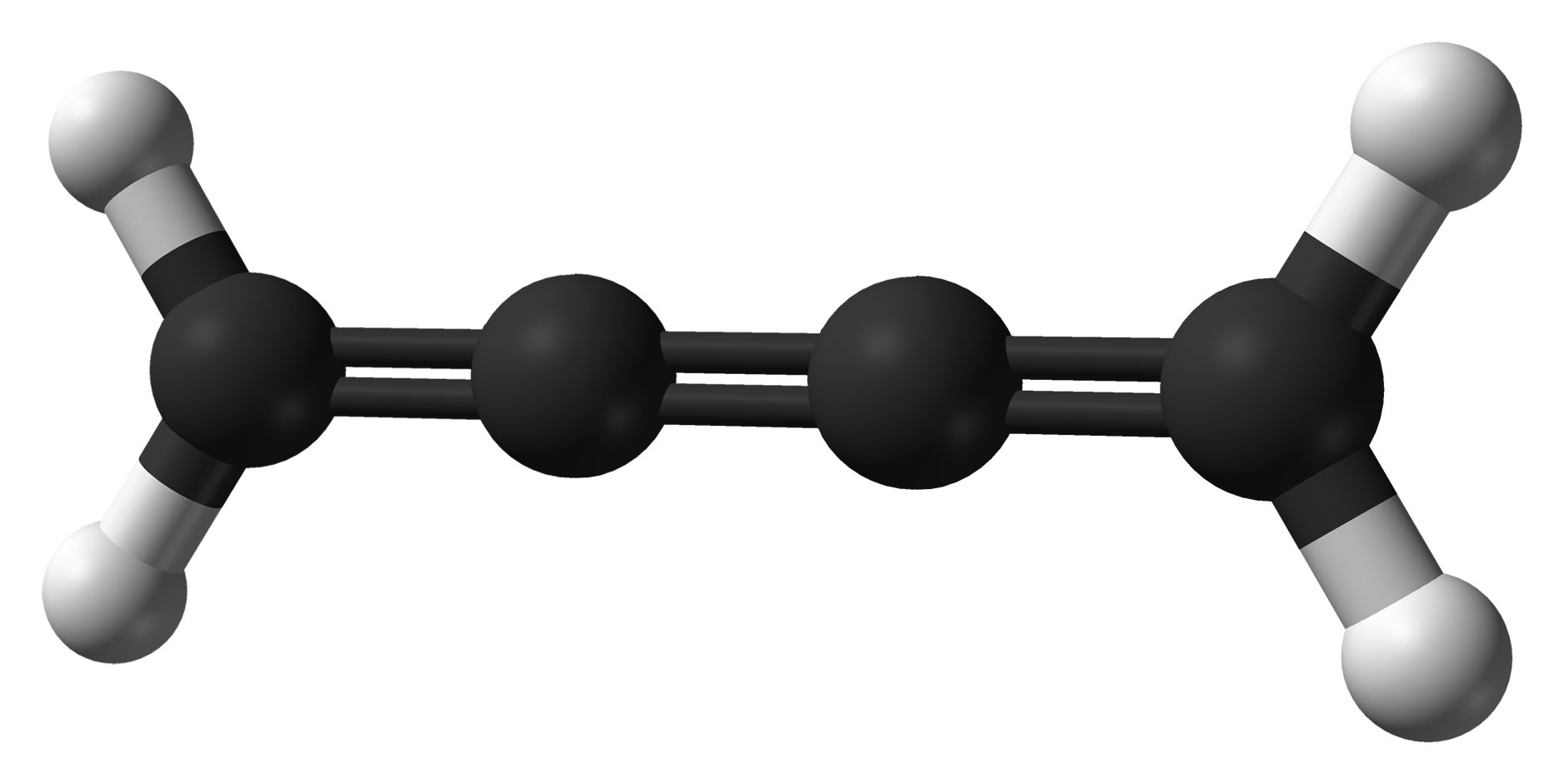
Cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular clouds and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalovinylidene. Tetraphenylbutatriene was reported synthesized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order of three. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as dinitrogen and carbon monoxide, are also triple bonded. In skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding The types of bonding can be explained in terms of orbital hybridization. In the case of acetylene each carbon atom has two sp-orbitals and two p-orbitals. The two sp-orbitals are linear with 180° angles and occupy the x-axis ( cartesian coordinate system). The p-orbitals are perpendicular on the y-axis and the z-axis. When the carbon atoms approach each other, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |