|
Cost Of Drug Development
The cost of drug development is the full cost of bringing a new drug (i.e., new chemical entity) to market from drug discovery through clinical trials to approval. Typically, companies spend tens to hundreds of millions of U.S. dollars on drug development. One element of the complexity is that the much-publicized final numbers often not only include the out-of-pocket expenses for conducting a series of Phase I-III clinical trials, but also the ''capital costs'' of the long period (10 or more years) during which the company must cover out-of-pocket costs for preclinical drug discovery. Additionally, companies often do not report whether a given figure includes the capitalized cost or comprises only out-of-pocket expenses, or both. One study assessed both capitalized and out-of-pocket costs as about US$1.8 billion and $870 million, respectively. In an analysis of the drug development costs for 98 companies over a decade, the average cost per drug developed and approved by a single ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Chemical Entity
A new chemical entity (NCE) is, according to the U.S. Food and Drug Administration, a novel, small, chemical molecule drug that is undergoing clinical trials or has received a first approval (not a new use) by the FDA in any other application submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act. A new molecular entity (NME) is a broader term that emcompasses both an NCE or an NBE (New Biological Entity). Definition An active moiety is a molecule or ion, excluding those appended portions of the molecule that cause the drug to be an ester, salt (including a salt with hydrogen or coordination bonds), or other noncovalent derivative (such as a complex, chelate, or clathrate) of the molecule, responsible for the physiological or pharmacological action of the drug substance. An NCE is a molecule developed by the innovator company in the early drug discovery stage, which after undergoing clinical trials could translate into a drug that could be a treatment for some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AstraZeneca
AstraZeneca plc () is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. It has been involved in developing the Oxford–AstraZeneca COVID-19 vaccine. The company was founded in 1999 through the merger of the Swedish Astra AB and the British Zeneca Group (itself formed by the demerger of the pharmaceutical operations of Imperial Chemical Industries in 1993). Since the merger it has been among the world's largest pharmaceutical companies and has made numerous corporate acquisitions, including Cambridge Antibody Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) and Definiens (by MedImmune in 2014). It has its research and development concentrated in three strategic centres: Cambridge, England; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medication Costs
Medication costs, also known as drug costs are a common health care cost for many people and health care systems. Prescription costs are the costs to the end consumer. Medication costs are influenced by multiple factors such as patents, stakeholder influence, and marketing expenses. A number of countries including Canada, parts of Europe, and Brasil use external reference pricing as a means to compare drug prices and to determine a base price for a particular medication. Other countries use pharmacoeconomics, which looks at the cost/benefit of a product in terms of quality of life, alternative treatments (drug and non-drug), and cost reduction or avoidance in other parts of the health care system (for example, a drug may reduce the need for a surgical intervention, thereby saving money). Structures like the UK's National Institute for Health and Clinical Excellence and to a lesser extent Canada's Common Drug Review (a division of the Canadian Agency for Drugs and Technologies in Heal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argument
An argument is a statement or group of statements called premises intended to determine the degree of truth or acceptability of another statement called conclusion. Arguments can be studied from three main perspectives: the logical, the dialectical and the rhetorical perspective. In logic, an argument is usually expressed not in natural language but in a symbolic formal language, and it can be defined as any group of propositions of which one is claimed to follow from the others through deductively valid inferences that preserve truth from the premises to the conclusion. This logical perspective on argument is relevant for scientific fields such as mathematics and computer science. Logic is the study of the forms of reasoning in arguments and the development of standards and criteria to evaluate arguments. Deductive arguments can be valid, and the valid ones can be sound: in a valid argument, premisses necessitate the conclusion, even if one or more of the premises is false ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Health Economics
The ''Journal of Health Economics'' is a peer-reviewed academic journal that publishes articles about health economics and related fields concerning human health care and medicine. The journal is published six times annually by Elsevier. The editors-in-chief are M. Alsan (Harvard Kennedy School), Anderson (University of California, Berkeley), A. Balsa (University of Montevideo), M.K. Bundorf (Stanford University), C. Carpenter (Vanderbilt University), J. Cawley (Cornell University), J.P. Clemens (University of California, San Diego), M. Kifmann (Universität Hamburg), M. Lindeboom (Vrije Universiteit Amsterdam), O.A. O'Donnell (Erasmus Universiteit), M. Shah (University of California at Los Angeles), L. Siciliani (University of York), and K. Simon (Indiana University). According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Drug
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tufts Center For The Study Of Drug Development
The Tufts Center for the Study of Drug Development is an independent, academic, non-profit research center at Tufts University in Boston, dedicated to researching drug development. It was established in 1976 by American physician Louis Lasagna. The Center develops and publishes information to help researchers, regulators, and policy makers in areas related to the pharmaceutical and biotechnology industries. In any given year, approximately 55% of Tufts CSDD's operating expenses are supported by grants from the private sector and 45% from the public sector. Research The Center studies trends in the pharmaceutical industry, maintaining databases pertaining to investigational new drugs, approved drugs, biopharmaceuticals, fast-tracked drugs, and orphan drugs. The Center provides this information with the aim to improve the efficiency of drug development, foster innovation, and increase patient access to medicines. Drug development costs The center has published numerous studies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
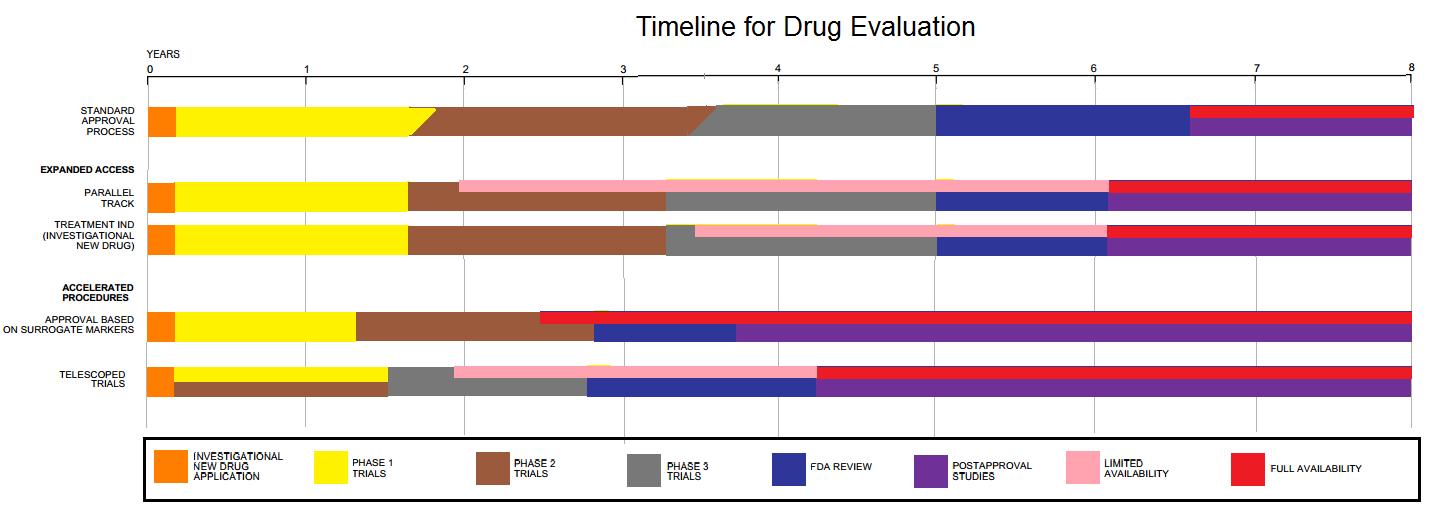
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered. Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy. Modern drug discovery involves the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered. Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy. Modern drug discovery involves the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_I_(cropped).jpg)

.jpg)