|
Cooperative Binding
Molecular binding is an interaction between molecules that results in a stable physical association between those molecules. Cooperative binding occurs in binding systems containing more than one type, or species, of molecule and in which one of the partners is not mono-valent and can bind more than one molecule of the other species. For example, consider a system where one molecule of species A can bind to molecules of species B. Species A is called the receptor and species B is called the ligand. Binding can be considered "cooperative" if the binding of the first molecule of B to A changes the binding affinity of the second B molecule, making it more or less likely to bind. In other words, the binding of B molecules to the different sites on A do not constitute mutually independent events. Cooperativity can be positive or negative. Cooperative binding is observed in many biopolymers, including proteins and nucleic acids. Cooperative binding has been shown to be the mechanism unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Binding
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding. In some cases, the associations can be quite strong—for example, the protein streptavidin and the vitamin biotin have a dissociation constant (reflecting the ratio between bound and free biotin) on the order of 10−14—and so the reactions are effectively irreversible. The result of molecular binding is sometimes the formation of a molecular complex in which the attractive forces holding the components together are generally non-covalent, and thus are normally energetically weaker than covalent bonds. Molecular binding occurs in biological complexes (e.g., between pairs or sets of proteins, or between a protein and a small molecule ligand it binds) and also in abiologic chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MWC Energy
MWC may refer to: * Mark Williams Company, a software company * '' Married... with Children'', a U.S. television situation comedy * Ma Wan Channel, a channel between Ma Wan and Tsing Yi islands in Hong Kong * Mennonite World Conference, a global community of Christian churches * Midwest Conference, a U.S. college athletic conference * The Minnesota Wrecking Crew, a Canadian sketch comedy troupe * Mobile World Congress, annual conference and trade show for the mobile phone industry in Barcelona * Monod-Wyman-Changeux model, biochemical model of protein transitions * Mountain West Conference, another U.S. collegiate athletic conference (more often abbreviated as MW) * Multiply-with-carry pseudorandom number generator, an algorithm * Music World Corporation, a U.S. music publishing company * Oklahoma Marginal Wells Commission Sustaining Oklahoma's Energy Resources (SOER), formerly known as the Oklahoma Commission on Marginally Producing Oil and Gas Well, and formerly commonly known as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively. Acetylcholine is the neurotransmitter used at the neuromuscular junction—in other words, it is the chemical that motor neurons of the nervous system release in order to activate muscles. This property means that drugs that affect cholinergic systems can have very dangerous effects ranging from paralysis to convulsions. Acetylcholine is also a neurotransmitter in the autonomic nervous system, both as an internal transmitter for the sympathetic nervous system and as the final product released by the parasymp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Receptors
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system. The nicotinic receptors are considered cholinergic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Lipscomb
William Nunn Lipscomb Jr. (December 9, 1919April 14, 2011) was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry. Biography Overview Lipscomb was born in Cleveland, Ohio. His family moved to Lexington, Kentucky in 1920, and he lived there until he received his Bachelor of Science degree in Chemistry at the University of Kentucky in 1941. He went on to earn his Doctor of Philosophy degree in Chemistry from the California Institute of Technology (Caltech) in 1946. From 1946 to 1959 he taught at the University of Minnesota. From 1959 to 1990 he was a professor of chemistry at Harvard University, where he was a professor emeritus since 1990. Lipscomb was married to the former Mary Adele Sargent from 1944 to 1983. They had three children, one of whom lived only a few hours. He married Jean Evans in 1983. They had one adopted daughter. Lipscomb resided in Cambridge, Massach ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
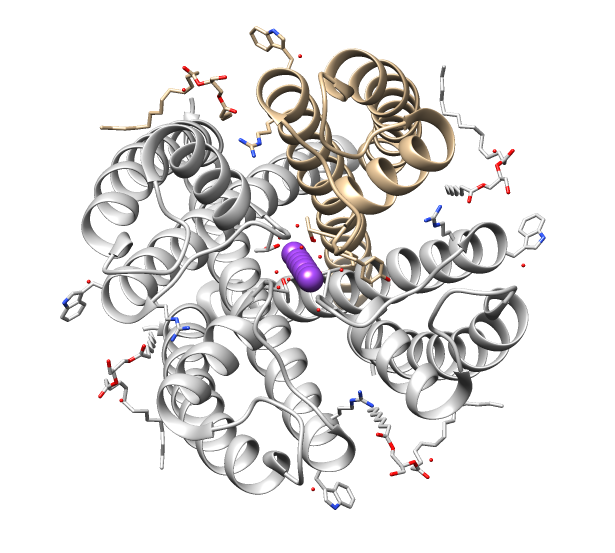
Aspartate Transcarbamoylase
Aspartate carbamoyltransferase (also known as aspartate transcarbamoylase or ATCase) catalyzes the first step in the pyrimidine biosynthetic pathway (). In ''E. coli'', the enzyme is a multi- subunit protein complex composed of 12 subunits (300 kDa in total). The composition of the subunits is C6R6, forming 2 trimers of catalytic subunits (34 kDa) and 3 dimers of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates. The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions. ATCase does not follow Michaelis–Menten kinetics. Instead, it lies between its low-activity, low-affinity "tense" and its high-activity, high-affinity "relaxed" states. The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory subun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine Ammonia-lyase
Threonine ammonia-lyase (EC 4.3.1.19, systematic name L-threonine ammonia-lyase (2-oxobutanoate-forming), also commonly referred to as threonine deaminase or threonine dehydratase, is an enzyme responsible for catalyzing the conversion of L-threonine into α-ketobutyrate and ammonia: :L-threonine = 2-oxobutanoate + NH3 (overall reaction) ::(1a) L-threonine = 2-aminobut-2-enoate + H2O ::(1b) 2-aminobut-2-enoate = 2-iminobutanoate (spontaneous) ::(1c) 2-iminobutanoate + H2O = 2-oxobutanoate + NH3 (spontaneous) α-Ketobutyrate can be converted into L-isoleucine, so threonine ammonia-lyase functions as a key enzyme in BCAA synthesis. It employs a pyridoxal-5'-phosphate cofactor, similar to many enzymes involved in amino acid metabolism. It is found in bacteria, yeast, and plants, though most research to date has focused on forms of the enzyme in bacteria. This enzyme was one of the first in which negative feedback inhibition by the end product of a metabolic pathway was directly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Perutz
Max Ferdinand Perutz (19 May 1914 – 6 February 2002) was an Austrian-born British molecular biologist, who shared the 1962 Nobel Prize for Chemistry with John Kendrew, for their studies of the structures of haemoglobin and myoglobin. He went on to win the Royal Medal of the Royal Society in 1971 and the Copley Medal in 1979. At Cambridge he founded and chaired (1962–79) The Medical Research Council (MRC) Laboratory of Molecular Biology (LMB), fourteen of whose scientists have won Nobel Prizes. Perutz's contributions to molecular biology in Cambridge are documented in ''The History of the University of Cambridge: Volume 4 (1870 to 1990)'' published by the Cambridge University Press in 1992. Early life and education Perutz was born in Vienna, the son of Adele "Dely" (Goldschmidt) and Hugo Perutz, a textile manufacturer. His parents were Jewish by ancestry, but had baptised Perutz in the Catholic religion. Although Perutz rejected religion and was an atheist in his later year ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Modulator
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a Receptor (biochemistry), receptor to change that receptor's response to stimulus. Some of them, like benzodiazepines, are drugs. The site that an allosteric modulator binds to (i.e., an ''allosteric site'') is not the same one to which an endogenous agonist of the receptor would bind (i.e., an ''orthosteric site''). Modulators and agonists can both be called receptor Ligand (biochemistry), ligands. Allosteric modulators can be 1 of 3 types either: positive, negative or neutral. Positive types increase the response of the receptor by increasing the probability that an agonist will bind to a receptor (i.e. Affinity (pharmacology), affinity), increasing its ability to activate the receptor (i.e. Efficacy (pharmacology), efficacy), or both. Negative types decrease the agonist affinity and/or efficacy. Neutral types don't affect agonist activity but can stop other modulators from binding to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |