|
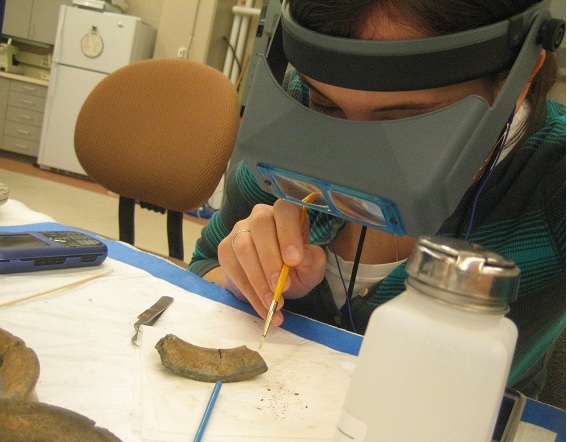
Conservation And Restoration Of Ceramic Objects
Conservation and restoration of ceramic objects is a process dedicated to the preservation and protection of objects of historical and personal value made from ceramic. Typically, this activity of conservation-restoration is undertaken by a conservator-restorer, especially when dealing with an object of cultural heritage. Ceramics are created from a production of coatings of inorganic, nonmetallic materials using heating and cooling to create a glaze. These coatings are often permanent and sustainable for utilitarian and decorative purposes. . Ctioa.org. Retrieved on 2012-03-28. The cleaning, handling, storage, and in general treatment of ceramics is consistent with that of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramics Conservation
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were fired clay bricks used for building house walls and other structures. Other pottery objects such as pots, vessels, vases and figurines were made from clay, either by itself or mixed with other materials like silica, hardened by sintering in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial, and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as semiconductors. The word ''ceramic'' comes from the Ancient Greek word (), meaning "of o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vandalism
Vandalism is the action involving deliberate destruction of or damage to public or private property. The term includes property damage, such as graffiti and defacement directed towards any property without permission of the owner. The term finds its roots in an Enlightenment view that the Germanic Vandals were a uniquely destructive people, as they sacked Rome in 455 AD. Etymology The Vandals, an ancient Germanic people, are associated with senseless destruction as a result of their sack of Rome under King Genseric in 455. During the Enlightenment, Rome was idealized, while the Goths and Vandals were blamed for its destruction. The Vandals may not have been any more destructive than other invaders of ancient times, but they did inspire English poet John Dryden to write, ''Till Goths, and Vandals, a rude Northern race, Did all the matchless Monuments deface'' (1694). However, the Vandals did intentionally damage statues, which may be why their name is associated with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bubble Wrap
Bubble wrap is a pliable transparency (optics), transparent plastic material commonly used for protecting fragile items during shipping. Known for its cushioning air-filled bubbles, it has also become a cultural icon, celebrated for its satisfying popping sound and alternative uses as a stress-relief tool. Regularly spaced, protruding air-filled hemispheres (bubbles) provide cushioning for fragile items. In 1957, two inventors named Alfred Fielding and Marc Chavannes were attempting to create a three-dimensional plastic wallpaper. Although the idea was a failure, they found that what they made could be used as packing material. Sealed Air was co-founded by Fielding in 1960. The term "bubble wrap" is owned by Sealed Air Corporation, but has become a generic trademark. Similar product names include bubble pack,The term "bubble pack" can also refer to a blister pack. air bubble packing, bubble wrapping and aeroplast. Design The bubbles that provide the cushioning for fragile or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'' and many variations thereof. Its properties can be modified further by crosslinking or copolymerization. All forms are nontoxic as well as chemically resilient, contributing to polyethylene's popularity as a multi-use plastic. However, polyethylene's chemical resilience also makes it a long-lived and decomposition-resistant pollutant when disposed of improperly. Being a h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallize
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as ammonium () and carbonate () ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, such as sodium hydroxide and potassium oxide. Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures when solid. Sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Bicarbonate
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (), and carbonate () ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water. All waters in contact with the atmosphere absorb carbon dioxide, and as these waters come into contact with rocks and sediments they acquire metal ions, most commonly calcium and magnesium, so most natural waters that come from streams, lakes, and especially wells, can be regarded as dilute solutions of these bicarbonates. These hard waters tend to form carbonate scale in pipes and boilers, and they react with soaps to form an undesirable scum. Attempts to prepare compounds such as solid calcium bicarbonate by evaporating its solution t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German , a term from the 19th century that came from the Latin word for Lime (material), lime, (genitive ) with the suffix ''-ite'' used to name minerals. It is thus a Doublet (linguistics), doublet of the word ''wikt:chalk, chalk''. When applied by archaeology, archaeologists and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also Crystallization, crystallizes as translucent crystals of selenite (mineral), selenite. It forms as an evaporite mineral and as a Mineral hydration, hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on Scratch hardness, scratch hardness comparison. Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Etymology and history The word ''wikt:gypsum, gypsum'' is derived from the Greek language, Greek word (), "plaster". Because the quarry, quarries of the Montmartre district of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystals
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |