|
Compliance Constants
Compliance Constants are the elements of an inverted Hessian matrix. The calculation of compliance constants provides an alternative description of chemical bonds in comparison with the widely used force constants explicitly ruling out the dependency on the coordinate system. They provide the unique description of the mechanical strength for covalent and non-covalent bonding. While force constants (as energy second derivatives) are usually given in a J/Å or N/cm, compliance constants are given in Å/a J or Å/ mdyn. History Hitherto, recent publications that broke the wall of putative chemical understanding and presented detection/isolation of novel compounds with intriguing bonding characters can still be provocative at times. The stir in such discoveries arose partly from the lack of a universally accepted bond descriptor. While bond dissociation energies (BDE) and rigid force constants have been generally regarded as primary tools for such interpretation, they are prone t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hessian Matrix
In mathematics, the Hessian matrix or Hessian is a square matrix of second-order partial derivatives of a scalar-valued function, or scalar field. It describes the local curvature of a function of many variables. The Hessian matrix was developed in the 19th century by the German mathematician Ludwig Otto Hesse and later named after him. Hesse originally used the term "functional determinants". Definitions and properties Suppose f : \R^n \to \R is a function taking as input a vector \mathbf \in \R^n and outputting a scalar f(\mathbf) \in \R. If all second-order partial derivatives of f exist, then the Hessian matrix \mathbf of f is a square n \times n matrix, usually defined and arranged as follows: \mathbf H_f= \begin \dfrac & \dfrac & \cdots & \dfrac \\ .2ex \dfrac & \dfrac & \cdots & \dfrac \\ .2ex \vdots & \vdots & \ddots & \vdots \\ .2ex \dfrac & \dfrac & \cdots & \dfrac \end, or, by stating an equation for the coefficients using indices i and j, (\mathbf H_f)_ = \fra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
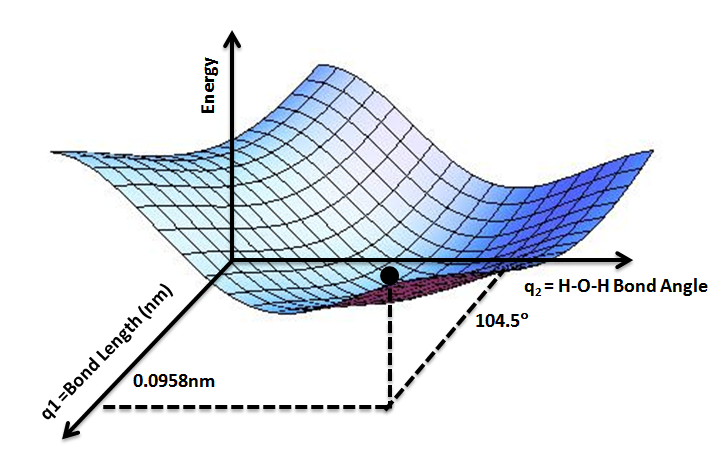
Potential Energy Surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential. It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground). The PES concept finds application in fields such as chemistry and physics, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lower melting point of , well below the freezing point of water, is claimed for the alloy galinstan (62–� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
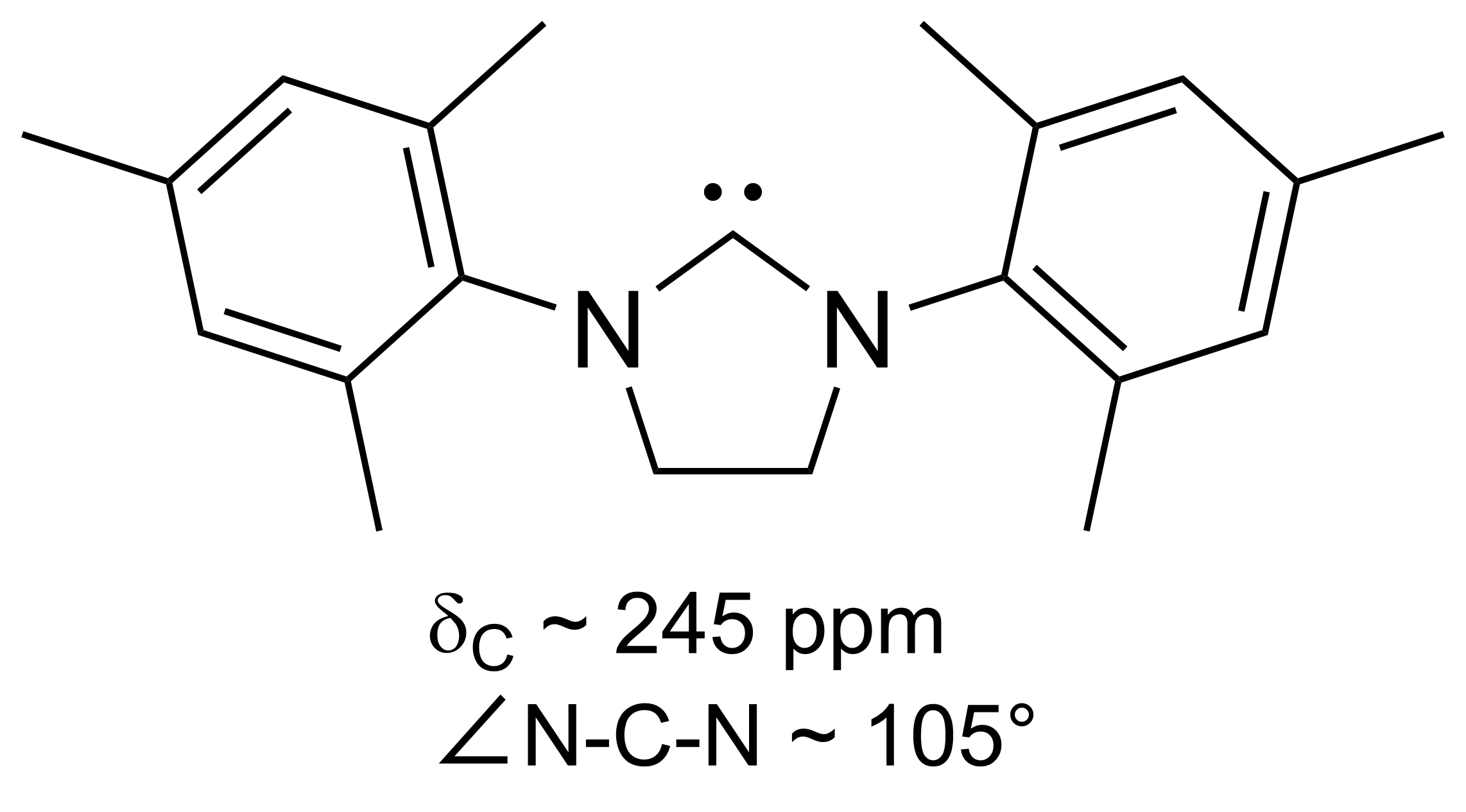
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diboryne
A diboryne in chemistry is a chemical compound containing a boron–boron triple bond. Such compounds are of fundamental importance in the study of chemical bonding, though only few have been reported. A diboryne stabilized by two carbon monoxide groups, (OC)B≡B(CO), was reported isolated in matrix isolation in 2002. A diboryne stable at room temperature with two ''N''-heterocyclic carbene (NHC) units was reported by Holger Braunschweig et al. in 2012. In terms of qualitative molecular orbital theory In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecul ..., the B2 molecule itself is expected to have a single bond, but with NHC ligands, the third excited state yields a triple bond. See also * Diborene References {{reflist Boron compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane - New
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-butane - New
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered by the chemist Dr. Walter Snelling in 1912. It was found dissolved in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties. Butane is one of a group of liquefied petroleum gases (LP gases). The others include propane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Butane burns more cleanly than gasoline and coal. Density The density of butane is highly dependent on temperature and pressure in the reservoir. For example, the density of liquid phase is 571.8±1 kg/m3 (for pressures up to 2MPa and temperature 27±0.2 °C), while the density of liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butane
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered by the chemist Dr. Walter Snelling in 1912. It was found dissolved in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties. Butane is one of a group of liquefied petroleum gases (LP gases). The others include propane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Butane burns more cleanly than gasoline and coal. Density The density of butane is highly dependent on temperature and pressure in the reservoir. For example, the density of liquid phase is 571.8±1 kg/m3 (for pressures up to 2MPa and temperature 27±0.2 °C), while the density of liq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |