|
Complex I
Respiratory complex I, (also known as NADH:ubiquinone oxidoreductase, Type I NADH dehydrogenase and mitochondrial complex I) is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes the transfer of electrons from NADH to coenzyme Q10 (CoQ10) and translocates protons across the inner mitochondrial membrane in eukaryotes or the plasma membrane of bacteria. This enzyme is essential for the normal functioning of cells, and mutations in its subunits lead to a wide range of inherited neuromuscular and metabolic disorders. Defects in this enzyme are responsible for the development of several pathological processes such as ischemia/reperfusion damage (stroke and cardiac infarction), Parkinson's disease and others. Function Complex I is the first enzyme of the mitochondrial electron transport chain. There are three energy-transducing enzymes in the electron transport chain - NADH:ubiquinone oxidoreductase (comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH Dehydrogenase Mechanism (Fixed)
Nicotinamide adenine dinucleotide (NAD) is a Cofactor (biochemistry), coenzyme central to metabolism. Found in all living cell (biology), cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other, nicotinamide. NAD exists in two forms: an Redox, oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In cellular metabolism, NAD is involved in redox reactions, carrying electrons from one reaction to another, so it is found in two forms: NAD is an oxidizing agent, accepting electrons from other molecules and becoming reduced; with H+, this reaction forms NADH, which can be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. It is also used in other cellular processes, most notably as a substrate (biochemistry), substrate of enzymes in adding or removing chemical groups to or fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer. When consumed in a Metabolism, metabolic process, ATP converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. It is also a Precursor (chemistry), precursor to DNA and RNA, and is used as a coenzyme. An average adult human processes around 50 kilograms (about 100 mole (unit), moles) daily. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of three parts: a sugar, an amine base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquinol
A ubiquinol is an electron-rich (reduced) form of coenzyme Q (ubiquinone). The term most often refers to ubiquinol-10, with a 10-unit tail most commonly found in humans. The natural ubiquinol form of coenzyme Q is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side-chain is 9-10 units long in mammals. Coenzyme Q10 (CoQ10) exists in three redox states, fully oxidized (ubiquinone), partially Redox, reduced (semiquinone or ubisemiquinone), and fully reduced (ubiquinol). The redox functions of ubiquinol in Bioenergetics, cellular energy production and antioxidant protection are based on the ability to exchange two electrons in a redox cycle between ubiquinol (reduced) and the ubiquinone (oxidized) form. Characteristics Because humans can synthesize ubiquinol, it is not classed as a vitamin. Bioavailability CoQ10 is not well absorbed into the body. Since the ubiquinol form has two additional hydrogens, it results in the conversion of two ketone groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquinone
Coenzyme Q10 (CoQ10 ), also known as ubiquinone, is a naturally occurring Cofactor (biochemistry), biochemical cofactor (coenzyme) and an antioxidant produced by the human body. It can also be obtained from dietary sources, such as meat, fish, seed oils, vegetables, and dietary supplements. CoQ10 is found in many organisms, including animals and bacteria. CoQ10 plays a role in mitochondrial oxidative phosphorylation, aiding in the production of adenosine triphosphate (ATP), which is involved in energy transfer within cells. The structure of CoQ10 consists of a benzoquinone moiety and an isoprenoid side chain, with the "10" referring to the number of Isoprene, isoprenyl chemical subunits in its tail. Although a ubiquitous molecule in human tissues, CoQ10 is not a dietary nutrient and does not have a Dietary Reference Intake, recommended intake level, and its use as a supplement is not approved drug, approved in the United States for any health or anti-disease effect. Biologica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
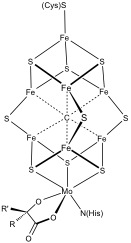
Iron–sulfur Protein
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, formin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Adenine Dinucleotide
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex. FAD can exist in four redox states, which are the flavin-N(5)-oxide, quinone, semiquinone, and hydroquinone. FAD is converted between these states by accepting or donating electrons. FAD, in its fully oxidized form, or quinone form, accepts two electrons and two protons to become FADH2 (hydroquinone form). The semiquinone (FADH·) can be formed by either reduction of FAD or oxidation of FADH2 by accepting or donating one electron and one proton, respectively. Some proteins, however, generate and maintain a super ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Mononucleotide
Flavin mononucleotide (FMN), or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as the prosthetic group of various oxidoreductases, including NADH dehydrogenase, as well as a cofactor in biological blue-light photo receptors. During the catalytic cycle, various oxidoreductases induce reversible interconversions between the oxidized (FMN), semiquinone (FMNH•), and reduced (FMNH2) forms of the isoalloxazine core. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. In its role as blue-light photo receptor, (oxidized) FMN stands out from the 'conventional' photo receptors as the signaling state and not an E/Z isomerization. It is the principal form in which riboflavin is found in cells and tissues. It requires more energy to produce, but is more soluble than riboflavin. In cells, FMN occurs freely circulating but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP Synthase
ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is: * ADP + Pi + 2H+out ATP + H2O + 2H+in ATP synthase lies across a cellular membrane and forms an aperture that hydron (chemistry), protons can cross from areas of high concentration to areas of low concentration, imparting energy for the synthesis of ATP. This electrochemical gradient is generated by the electron transport chain and allows cells to store energy in ATP for later use. In prokaryote, prokaryotic cells ATP synthase lies across the plasma membrane, while in eukaryote, eukaryotic cells it lies across the inner mitochondrial membrane. Organisms capable of photosynthesis also have ATP synthase across the thylakoid membrane, which in plants is located in the chloroplast and in cyanobacteria is located in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mrp Superfamily
The Na+ Transporting Mrp Superfamily is a superfamily of integral membrane transport proteins. It includes the TC families:2.A.63- The Monovalent Cation (K+ or Na+):Proton Antiporter-3 (CPA3) Family3.D.1- The H+ or Na+-translocating NADH Dehyrogenase (NDH) Family3.D.9- The H+-translocating F420H2 Dehydrogenase (F420H2DH) Family Mrp of ''Bacillus subtilis'' is a 7 subunit Na+/H+ antiporter An antiporter (also called exchanger or counter-transporter) is an integral membrane protein that uses secondary active transport to move two or more molecules in opposite directions across a phospholipid membrane. It is a type of cotransporte ... comple(TC# 2.A.63.1.4) All subunits are homologous to the subunits in other members of this monovalent cation (K+ or Na+):proton antiporter-3 (CPA3) family as well as subunits in the archaeal hydrogenasesTC#s 3.D.1.4.1an3.D.1.4.2, which share several subunits with NADH dehydrogenase subunits (3.D.1). The largest subunits of the Mrp complex (Mrp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Programmed Cell Death
Programmed cell death (PCD) sometimes referred to as cell, or cellular suicide is the death of a cell (biology), cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers advantage during an organism's biological life cycle, lifecycle. For example, the Limb development, differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and animal tissue development. Apoptosis and autophagy are both forms of programmed cell death. Necrosis is the death of a cell caused by external factors such as trauma or infection and occurs in several different forms. Necrosis was long seen as a non-physiological process that occurs as a result of infection or injury, but in the 2000s, a form of programmed necrosis, called necroptosis, was recognized as an alternative f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biology), morphology) and death. These changes include Bleb (cell biology), blebbing, Plasmolysis, cell shrinkage, Karyorrhexis, nuclear fragmentation, Pyknosis, chromatin condensation, Apoptotic DNA fragmentation, DNA fragmentation, and mRNA decay. The average adult human loses 50 to 70 1,000,000,000, billion cells each day due to apoptosis. For the average human child between 8 and 14 years old, each day the approximate loss is 20 to 30 billion cells. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodothermus Marinus
''Rhodothermus marinus'' is a species of bacteria. It is obligately aerobic, moderately halophilic, thermophilic, Gram-negative and rod-shaped, about 0.5 μm The micrometre (Commonwealth English as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American English), also commonly known by the non-SI term micron, is a unit of length in the International System ... in diameter and 2-2.5 μm long. References Further reading * * * External linksLPSN Bacteria described in 1995 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |