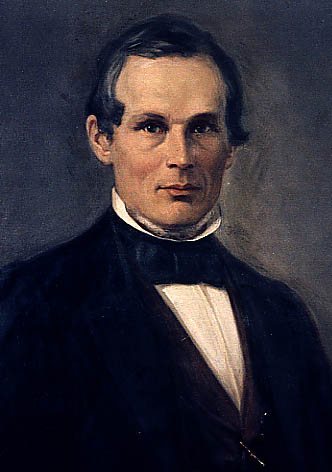|
Coloradoite
Coloradoite, also known as mercury telluride (HgTe), is a rare Telluride (chemistry), telluride ore associated with metallic deposit (especially gold and silver). Gold usually occurs within tellurides, such as coloradoite, as a high-finess native metal. The quest for mining led to the discovery of telluride ores which were found to be associated with metals. Tellurides are ingrown into ores containing these precious metals and are also responsible for a significant amount of these metals being produced. Coloradoite, a member of the coordination subclass of tellurides, is a covalent compound that is isostructural with sphalerite (ZnS).Povarennykh, A. S (1972). ''Crystal Chemical classification of minerals''. Vol I, pp. 120–121 Its chemical properties are highly instrumental in distinguishing it from other tellurides. It was first discovered in Colorado in 1877. Since then, other deposits have been found. Although it plays an important role in the geology of minerals, it can also b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Telluride Mineral
A telluride mineral is a mineral that has the telluride anion as a main component. Tellurides are similar to sulfides and are grouped with them in both the Dana and Strunz mineral classification systems.http://webmineral.com/strunz/II.shtml Webmineral Strunz Examples include: * altaite * calaverite * coloradoite * empressite * hessite * kostovite * krennerite * melonite * merenskyite * petzite * rickardite * stützite * sylvanite * tellurobismuthite * temagamite * tetradymite * vulcanite See also * Cripple Creek & Victor Gold Mine The Cripple Creek & Victor Gold Mine, formerly and historically the Cresson Mine, is an active gold mine located near the town of Victor, in the Cripple Creek mining district in the US state of Colorado. The richest gold mine in Colorado histo ... References * {{Mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Telluride
Mercury telluride (HgTe) is a binary chemical compound of mercury and tellurium. It is a semi-metal related to the II-VI group of semiconductor materials. Alternative names are mercuric telluride and mercury(II) telluride. HgTe occurs in nature as the mineral form coloradoite. Physical properties All properties are at standard temperature and pressure unless stated otherwise. The lattice parameter is about 0.646 nm in the cubic crystalline form. The bulk modulus is about 42.1 GPa. The thermal expansion coefficient is about 5.2×10−6/K. Static dielectric constant 20.8, dynamic dielectric constant 15.1. Thermal conductivity is low at 2.7 W·m2/(m·K). HgTe bonds are weak leading to low hardness values. Hardness 2.7×107 kg/m2. Doping N-type doping can be achieved with elements such as boron, aluminium, gallium, or indium. Iodine and iron will also dope n-type. HgTe is naturally p-type due to mercury vacancies. P-type doping is also achieved by introducing zinc, copper, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Augustus Genth
Frederick Augustus Ludwig Karl Wilhelm Genth (May 17, 1820 – February 2, 1893) was a German-American chemist, specializing in analytical chemistry and mineralogy. Biography Frederick Augustus Genth was born in Wächtersbach, Hesse-Cassel on May 17, 1820. He studied at the Hanau gymnasium and at the University of Heidelberg, under Justus von Liebig at Giessen, and finally under Christian Gerling (physics) and Robert Bunsen (chemistry) at Marburg, where he received the degree of Ph.D. in 1846. For three years (1845-1848) he acted as assistant to Bunsen. In 1848, Genth emigrated to the United States. He settled in Philadelphia and organized an analytical laboratory. In 1872 he was appointed professor of chemistry and mineralogy in the University of Pennsylvania. He resigned his professorship in 1888, and re-established his laboratory. He also held the office of chemist to the Geological Survey of Pennsylvania and also to the board of agriculture of that state. Genth was a mem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binary Compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended solids. Famous examples zinc sulfide, which contains zinc and sulfur, and tungsten carbide Tungsten carbide (chemical formula: WC) is a chemical compound (specifically, a carbide) containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed int ..., which contains tungsten and carbon. Phases with higher degrees of complexity feature more elements, e.g. three elements in ternary phases, four elements in quaternary phases. References Chemical compounds {{chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kirkland Lake
Kirkland Lake is a town and municipality in Timiskaming District in Northeastern Ontario, Canada. The 2016 population, according to Statistics Canada, was 7,981. The community name was based on a nearby lake which in turn was named after Winnifred Kirkland, a secretary of the Ontario Department of Mines in Toronto. The lake was named by surveyor Louis Rorke in 1907. Miss Kirkland never visited the town, and the lake that bore her name no longer exists because of mine tailings. The community comprises Kirkland Lake (Teck Township), as well as Swastika, Chaput Hughes, Bernhardt, and Morrisette Twp. Kirkland Lake was built on gold, but it is equally well known for producing world-famous hockey players. Indeed, legendary hockey broadcaster Foster Hewitt called Kirkland Lake "the town that made the NHL." The town celebrated this via Hockey Heritage North which has been renamed in the meantime to Heritage North. Until January 1, 1972, the town was known as Township of Teck. A by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smuggler Mine
The Smuggler Mine is located on the slopes of Smuggler Mountain, on the north edge of Aspen, Colorado, United States. It is the oldest operating silver mine in the Aspen mining district, and one of the few still operating from Aspen's early boom years. In 1987 it was listed on the National Register of Historic Places. The largest silver nugget ever mined, weighing , came from Smuggler. At its peak the mine was responsible for nearly one-fifth of the world's total silver output. Its extensive tunnel system reaches more than a thousand feet (300 m) below the entrance, extending under the city of Aspen, although most of the lower tunnels are presently flooded. Smuggler was one of the few mines in the Aspen area to reopen after the 1893 repeal of the Sherman Silver Purchase Act. It continued producing ore until 1918, and was reopened in the 1970s. In 1984 it was designated a Superfund site after tests found high levels of lead and cadmium in the soil. It took the Environmental Prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the ''precipitant''. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'supernate' or 'supernatant'. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (''e.g.'', metallurgy and alloys) when solid impurities segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angstrom
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ångström is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is Å, a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fracture
Fracture is the separation of an object or material into two or more pieces under the action of stress. The fracture of a solid usually occurs due to the development of certain displacement discontinuity surfaces within the solid. If a displacement develops perpendicular to the surface, it is called a normal tensile crack or simply a crack; if a displacement develops tangentially, it is called a shear crack, slip band or dislocation. Fracture#Brittle, Brittle fractures occur with no apparent deformation before fracture. Fracture#Ductile, Ductile fractures occur after visible deformation. Fracture strength, or breaking strength, is the stress when a specimen fails or fractures. The detailed understanding of how a fracture occurs and develops in materials is the object of fracture mechanics. Strength Fracture strength, also known as breaking strength, is the stress at which a specimen Structural integrity and failure, fails via fracture. This is usually determined for a gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallic Bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be described as the sharing of ''free'' electrons among a structure of positively charged ions (cations). Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster. Metallic bonding is not the only type of chemical bonding a metal can exhibit, even as a pure substance. For example, elemental gallium consists of covalently-bound pairs of atoms in both liquid and solid-state—these pairs form a crystal structure with metallic bonding between them. Another example of a metal–metal covalent bond is the mercurous ion (). History As chemistry developed into a science, it became clear that metals formed the majority of the periodic tabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |