|
Colobopsis Saundersi
''Colobopsis saundersi'', synonym ''Camponotus saundersi'', is a species of ant found in Malaysia and Brunei, belonging to the genus ''Colobopsis''. A worker can explode suicidally and aggressively as an ultimate act of defense, an ability it has in common with several other species in this genus and a few other insects. The ant has an enormously enlarged mandibular (jaw) gland, many times the size of a normal ant, which produces defense adhesive secretions. According to a 2018 study, this species forms a species complex and is probably related to '' C. explodens'', which is part of the '' C. cylindrica'' group. Defenses Its defensive behaviours include self-destruction by autothysis, a term coined by Maschwitz and Maschwitz (1974). Two oversized, poison-filled mandibular glands run the entire length of the ant's body. When combat takes a turn for the worse, the worker ant violently contracts its abdominal muscles to rupture its gaster at the intersegmental fold, which also bur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carlo Emery
Carlo Emery (25 October 1848, Naples – 11 May 1925) was an Italian entomologist. He is remembered for Emery's rule, which states that insect social parasitism (biology), social parasites are often closely related to their hosts. Early in his career Carlo Emery pursued a course in general medicine, and in 1872 narrowed his interests to ophthalmology. In 1878 he was appointed Professor of Zoology at the University of Cagliari, remaining there for several years until 1881 when he took up an appointment at the University of Bologna as Professor of Zoology, remaining there for thirty-five years until his death. Emery specialised in Hymenoptera, but his early work was on Coleoptera. Prior to 1869, his earliest works were a textbook of general zoology and papers on fishes and molluscs. From 1869 to 1925 he devoted himself almost entirely to the study of ants. Emery published extensively between 1869 and 1926 describing 130 genera and 1057 species mainly in Philogène Auguste Gali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
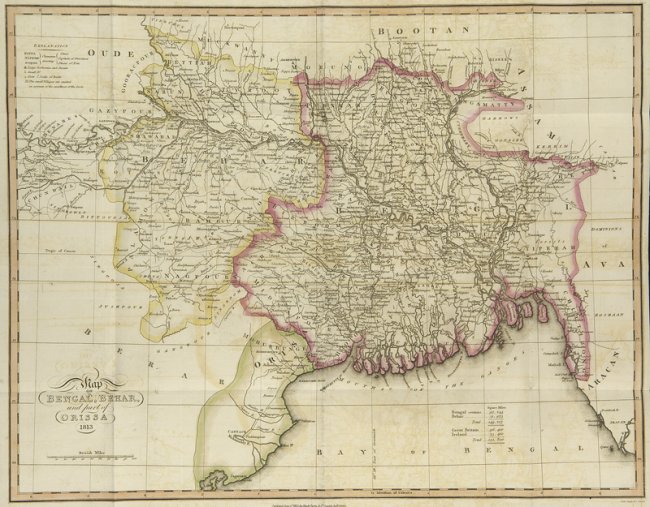
Dhaka
Dhaka ( or ; bn, ঢাকা, Ḍhākā, ), formerly known as Dacca, is the capital and largest city of Bangladesh, as well as the world's largest Bengali-speaking city. It is the eighth largest and sixth most densely populated city in the world with a population of 8.9 million residents as of 2011, and a population of over 21.7 million residents in the Greater Dhaka Area. According to a Demographia survey, Dhaka has the most densely populated built-up urban area in the world, and is popularly described as such in the news media. Dhaka is one of the major cities of South Asia and a major global Muslim-majority city. Dhaka ranks 39th in the world and 3rd in South Asia in terms of urban GDP. As part of the Bengal delta, the city is bounded by the Buriganga River, Turag River, Dhaleshwari River and Shitalakshya River. The area of Dhaka has been inhabited since the first millennium. An early modern city developed from the 17th century as a provincial capital and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citronellal
Citronellal or rhodinal ( C10 H18 O) is a monoterpenoid aldehyde, the main component in the mixture of terpenoid chemical compounds that give citronella oil its distinctive lemon scent. Citronellal is a main isolate in distilled oils from the plants ''Cymbopogon'' (excepting C. citratus, culinary lemongrass), lemon-scented gum, and lemon-scented teatree. The (''S'')-(−)-enantiomer of citronellal makes up to 80% of the oil from kaffir lime leaves and is the compound responsible for its characteristic aroma. Citronellal has insect repellent properties, and research shows high repellent effectiveness against mosquitoes. Another research shows that citronellal has strong antifungal qualities. Compendial status * British Pharmacopoeia See also * Citral * Citronellol * Citronella oil * Hydroxycitronellal * Perfume allergy Perfume intolerance or perfume allergy is a condition wherein people exhibit sensitivity or allergic reactions to ingredients in some perfumes and some oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant '' Salvia di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Undecane
Undecane (also known as hendecane) is a liquid alkane hydrocarbon with the chemical formula CH3(CH2)9CH3. It is used as a mild sex attractant for various types of moths and cockroaches, and an alert signal for a variety of ants.Hölldobler B, Wilson EO (1990). ''The Ants''. Harvard University Press. , p. 287 It has 159 isomers. Undecane may also be used as an internal standard in gas chromatography when working with other hydrocarbons. Since the boiling point of undecane (196 °C) is well known, it may be used as a comparison for retention times in a gas chromatograph for molecules whose structure has been freshly elucidated. For example, if one is working with a 50 m crosslinked methyl silicone capillary column with an oven temperature increasing slowly, beginning around 60 °C, an 11-carbon molecule like undecane may be used as an internal standard to be compared with the retention times of other 10-, 11-, or 12- carbon molecules, depending on their structures. Se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic Compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds ( alkynes). If other elements (heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. The least complex aliphatic compound is methane (CH4). Properties Most aliphatic compounds are flammable, allowing the use of hydrocarbons as fuel, such as methane in Bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orcinol
Orcinol is an organic compound with the formula CH3C6H3(OH)2. It occurs in many species of lichens including ''Roccella tinctoria'' and ''Lecanora''. Orcinol has been detected in the "toxic glue" of the ant species ''Camponotus saundersi''. It is a colorless solid. It is related to resorcinol, 1,3-C6H4(OH)2. Synthesis and reactions Orcinol was first prepared by dehydroacetic acid, a conversion that involved ring-opening of the pyrone to a triketone. This early experiment helped establish the rich condensation chemistry of polyketides. It can be obtained by fusing extract of aloes with potash, followed by acidification. It undergoes O-methylation with dimethylsulfate. It is used in the production of the dye orcein and as a reagent in some chemical tests for pentoses, such as Bial's Test. It may be synthesized from toluene; more interesting is its production when acetone dicarboxylic ester is condensed with the aid of sodium. It crystallizes in colorless prisms with one molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
M-cresol
''meta''-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of ''p''-cresol and ''o''-cresol. Production Together with many other compounds, ''m''-cresol is traditionally extracted from coal tar, the volatile materials obtained in the production of coke from (bituminous) coal. This residue contains a few percent by weight of phenol and isomeric cresols. In the cymene–cresol process, toluene is alkylated with propylene to give isomers of cymene, which can be oxidatively dealkylated analogous to the cumene process. Another method, involves carbonylation of a mixture of methallyl chloride and acetylene in the presence of nickel carbonyl. Applications ''m''-Cresol is a precursor to numerous compounds. Important applications include: * pesticides such as fenitrothion and fenthion * synthetic vita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insectes Sociaux
''Insectes sociaux'' is a scientific journal dedicated to the study of social insects. It is the official journal of the International Union for the Study of Social Insects (IUSSI), and is published by Birkhäuser Verlag Birkhäuser was a Swiss publisher founded in 1879 by Emil Birkhäuser. It was acquired by Springer Science+Business Media in 1985. Today it is an imprint used by two companies in unrelated fields: * Springer continues to publish science (particu .... References External links * Entomology journals and magazines Academic journals associated with international learned and professional societies Springer Science+Business Media academic journals {{zoo-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Appendages
An appendage (or outgrowth) is an external body part, or natural prolongation, that protrudes from an organism's body. In arthropods, an appendage refers to any of the homologous body parts that may extend from a body segment, including antennae, mouthparts (including mandibles, maxillae and maxillipeds), gills, locomotor legs (pereiopods for walking, and pleopods for swimming), sexual organs (gonopods), and parts of the tail (uropods). Typically, each body segment carries one pair of appendages. An appendage which is modified to assist in feeding is known as a maxilliped or gnathopod. In vertebrates, an appendage can refer to a locomotor part such as a tail, fins on a fish, limbs (legs, flippers or wings) on a tetrapod; exposed sex organ; defensive parts such as horns and antlers; or sensory organs such as auricles, proboscis ( trunk and snout) and barbels. Appendages may become ''uniramous'', as in insects and centipedes, where each appendage comprises a single series ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |