|
Coenzyme A Transferases
Coenzyme A transferases (CoA-transferases) are transferase enzymes that catalyze the transfer of a coenzyme A group from an acyl-CoA donor to a carboxylic acid acceptor. Among other roles, they are responsible for transfer of CoA groups during fermentation and metabolism of ketone bodies. These enzymes are found in all three domains of life (bacteria, eukaryotes, archaea). Reactions As a group, the CoA transferases catalyze 105 reactions at relatively fast rates. Some common reactions include :Acetyl-CoA + Butyrate \rightleftharpoons Acetate + Butyryl-CoA :Acetyl-CoA + Succinate \rightleftharpoons Acetate + Succinyl-CoA :Acetoacetate-CoA + Succinate \rightleftharpoons Acetoacetate + Succinyl-CoA :Formate + Oxalate \rightleftharpoons Formate + Oxalyl-CoA These reactions have different functions in cells. The reaction involving acetyl-CoA and butyrate (), for example, forms butyrate during fermentation. The reaction involving acetyl-CoA and succinate () is part o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
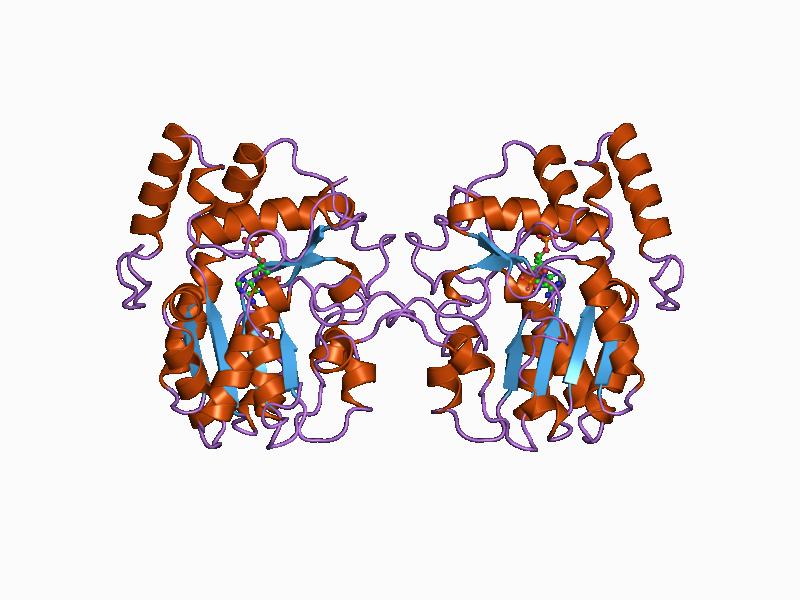
OXCT1
3-oxoacid CoA-transferase 1 (OXCT1) is an enzyme that in humans is encoded by the ''OXCT1'' gene. It is also known as succinyl-CoA-3-oxaloacid CoA transferase (SCOT). Mutations in the ''OXCT1'' gene are associated with succinyl-CoA:3-oxoacid CoA transferase deficiency. This gene encodes a member of the 3-oxoacid CoA-transferase gene family. The encoded protein is a homodimeric mitochondrial matrix enzyme that plays a central role in extrahepatic ketone body catabolism by catalyzing the reversible transfer of coenzyme A (CoA) from succinyl-CoA to acetoacetate. Structure Gene The ''OXCT1'' gene resides on chromosome 5 at the band 5p13. ''OXCT1'' spans a length of over 100 kb and includes 17 Exon, exons. Protein The crystal structure of human OXCT1 reveals it to be a homodimer with two Active site, active sites. Each of its Monomer, monomers contains N-terminus, N- and C-terminus, C-terminal domains that share an α/β structural fold characteristic of CoA transferase family I m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Catalysis
Enzyme catalysis is the increase in the rate of a process by a biological molecule, an "enzyme". Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, called the active site. Most enzymes are made predominantly of proteins, either a single protein chain or many such chains in a multi-subunit complex. Enzymes often also incorporate non-protein components, such as metal ions or specialized organic molecules known as cofactor (e.g. adenosine triphosphate). Many cofactors are vitamins, and their role as vitamins is directly linked to their use in the catalysis of biological process within metabolism. Catalysis of biochemical reactions in the cell is vital since many but not all metabolically essential reactions have very low rates when uncatalysed. One driver of protein evolution is the optimization of such catalytic activities, although only the most crucial enzymes operate near catalytic e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketoacidosis
Ketoacidosis is a metabolic state caused by uncontrolled production of ketone bodies that cause a metabolic acidosis. While ketosis refers to any elevation of blood ketones, ketoacidosis is a specific pathologic condition that results in changes in blood pH and requires medical attention. The most common cause of ketoacidosis is diabetic ketoacidosis but can also be caused by alcohol, medications, toxins, and rarely, starvation. Signs and symptoms The symptoms of ketoacidosis are variable depending on the underlying cause. The most common symptoms include nausea, vomiting, abdominal pain, and weakness. Breath may also develop the smell of acetone as it is a volatile ketone that can be exhaled. Rapid deep breathing, or Kussmaul breathing, may be present to compensate for the metabolic acidosis. Altered mental status is more common in diabetic than alcoholic ketoacidosis. Causes Ketoacidosis is caused by the uncontrolled production of ketone bodies. Usually the production of keton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinate—hydroxymethylglutarate CoA-transferase
In enzymology, a succinate-hydroxymethylglutarate CoA-transferase () is an enzyme that catalyzes the chemical reaction :succinyl-CoA + 3-hydroxy-3-methylglutarate \rightleftharpoons succinate + (S)-3-hydroxy-3-methylglutaryl-CoA Thus, the two substrates of this enzyme are succinyl-CoA and 3-hydroxy-3-methylglutarate, whereas its two products are succinate and (S)-3-hydroxy-3-methylglutaryl-CoA. This enzyme belongs to the family of transferase A transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of di ...s, specifically the CoA-transferases. The systematic name of this enzyme class is succinyl-CoA:3-hydroxy-3-methylglutarate CoA-transferase. Other names in common use include hydroxymethylglutarate coenzyme A-transferase, and dicarboxyl-CoA:dicarboxylic acid coenzyme A transferase. Ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-oxoacid CoA-transferase
In enzymology, a 3-oxoacid CoA-transferase () is an enzyme that catalyzes the chemical reaction :succinyl-CoA + a 3-oxo acid \rightleftharpoons succinate + a 3-oxoacyl-CoA Thus, the two substrates of this enzyme are succinyl-CoA and 3-oxo acid, whereas its two products are succinate and 3-oxoacyl-CoA. This enzyme belongs to the family of transferases, specifically the CoA-transferases. The systematic name of this enzyme class is succinyl-CoA:3-oxo-acid CoA-transferase. Other names in common use include 3-oxoacid coenzyme A-transferase, 3-ketoacid CoA-transferase, 3-ketoacid coenzyme A transferase, 3-oxo-CoA transferase, 3-oxoacid CoA dehydrogenase, acetoacetate succinyl-CoA transferase, acetoacetyl coenzyme A-succinic thiophorase, succinyl coenzyme A-acetoacetyl coenzyme A-transferase, and succinyl-CoA transferase. This enzyme participates in 3 metabolic pathways: synthesis and degradation of ketone bodies, valine, leucine and isoleucine degradation, and butanoate metabolis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Fold Class
In molecular biology, protein fold classes are broad categories of protein tertiary structure topology. They describe groups of proteins that share similar amino acid and secondary structure proportions. Each class contains multiple, independent protein superfamilies (i.e. are not necessarily evolutionarily related to one another). Generally recognised classes Four large classes of protein that are generally agreed upon by the two main structure classification databases (SCOP and CATH). all-α All-α proteins are a class of structural domains in which the secondary structure is composed entirely of α-helices, with the possible exception of a few isolated β-sheets on the periphery. Common examples include the bromodomain, the globin fold and the homeodomain fold. all-β All-β proteins are a class of structural domains in which the secondary structure is composed entirely of β-sheets, with the possible exception of a few isolated α-helices on the periphery. Common e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can just be considered as a single product resulting from the direct combination of different molecules which comprises all the reactant molecules' atoms. Adducts often form between Lewis acids and Lewis bases. A good example is the formation of adducts between the Lewis acid borane and the oxygen atom in the Lewis bases, tetrahydrofuran (THF): BH3·O(CH2)4 or diethyl ether: BH3·O(CH3CH2)2. Many Lewis acids and Lewis bases reacting in the gas phase or in non-aqueous solvents to form adducts have been examined in the ECW model. Trimethylboron, trimethyltin chloride and bis(hexaflu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketogenesis
Ketogenesis is the biochemical process through which organisms produce ketone bodies by breaking down fatty acids and ketogenic amino acids. The process supplies energy to certain organs, particularly the brain, heart and skeletal muscle, under specific scenarios including fasting, caloric restriction, sleep, or others. (In rare metabolic diseases, insufficient gluconeogenesis can cause excessive ketogenesis and hypoglycemia, which may lead to the life-threatening condition known as non-diabetic ketoacidosis.) Ketone bodies are not obligately produced from fatty acids; rather a meaningful amount of them is synthesized only in a situation of carbohydrate and protein insufficiency, where only fatty acids are readily available as fuel for their production. Production Ketone bodies are produced mainly in the mitochondria of liver cells, and synthesis can occur in response to an unavailability of blood glucose, such as during fasting. Other cells, e.g. human astrocytes, are capable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferase
A transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes. Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity of coenzyme A (CoA) transferase, which transfers thiol esters, the action of N-acetyltransferase, which is part of the pathway that metabolizes tryptophan, and the regulation of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl CoA. Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by a peptidyl transferase. The transfer involves the removal of the growing amino acid chain from the tRNA molecule in the A-site of the ribosome and its subse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |