|
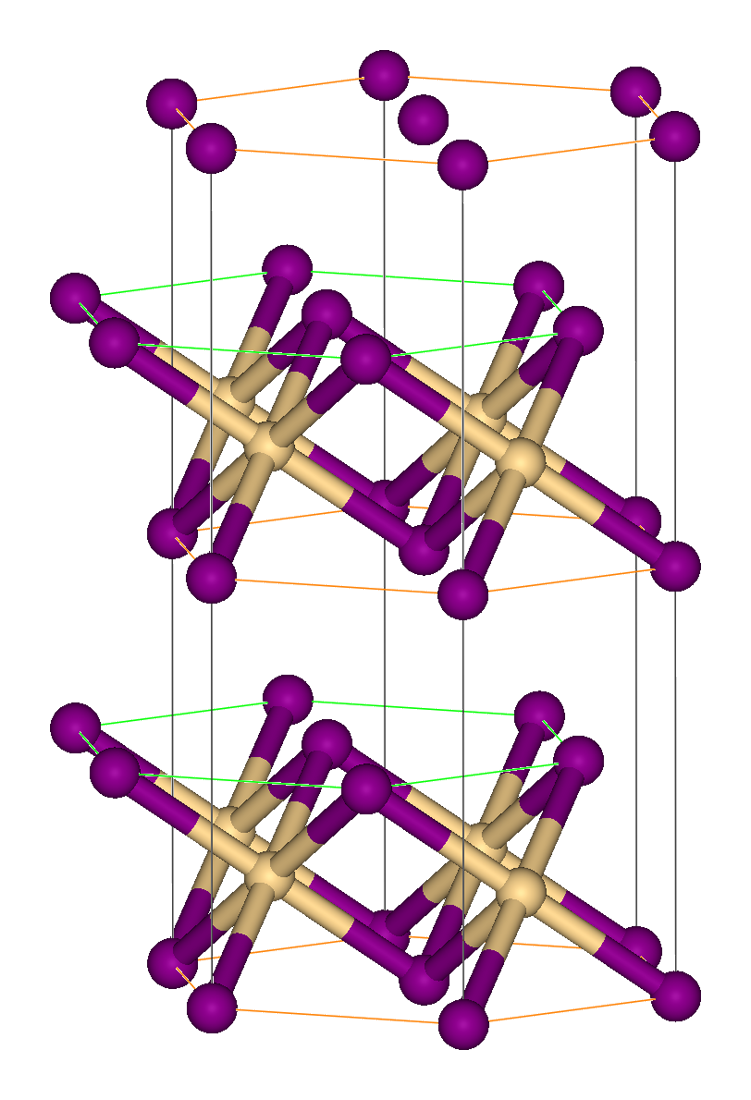
Cobalt(II) Cyanide
Cobalt(II) cyanide is the inorganic compound with the formula Co(CN)2. It is coordination polymer that has attracted intermittent attention over many years in the area of inorganic synthesis and homogeneous catalysis. Uses Cobalt(II) cyanide has been used as a precursor to dicobalt octacarbonyl. Preparation and structure The trihydrate salt is obtained as a reddish-brown precipitate by adding potassium cyanide to a cobalt salt solution: :CoCl2(H2O)6 + 2 KCN → Co(CN)2 + 2 KCl + 6 H2O Hydrated Co(CN)2 dissolves in the presence of excess potassium cyanide, forming a red solution of K''n''Co(CN)2+''n'' though it is disputed whether ''n''=3 or 4. This material further oxidizes to yellow hexacyanocobaltate(III), which can be isolated as the salt K3Co(CN)6. The solid is a coordination polymer consisting of cobalt atoms linked by cyanide units in a cubic arrangement, each such cobalt atom having octahedral geometry, and an additional cobalt atom in half of the cubic cavities. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Cyanide
Magnesium cyanide is a chemical compound with the formula Mg(CN)2. It is a toxic white solid. It has been theorized that it is a nitrile compound, but it has been disproved. If heated to 500 °C, it decomposes to magnesium nitride. Preparation The first attempt to prepare magnesium cyanide was attempted in 1924. It was attempted by reacting a solution of hydrogen cyanide in water with magnesium metal: :HCN + Mg → Mg(CN)2 + H2 However, no magnesium cyanide was observed, only magnesium hydroxide formed. To avoid this problem, instead of using water as the reaction medium, pure ammonia was used at -30 °C. This formed magnesium cyanide ammoniate, which in turn was heated to 180 °C to produce magnesium cyanide.A. R. Frank, R. B. Booth, American Cyanamid Co., US Patent 2419931, 1947. Other methods are possible, such as the decomposition of magnesium ferricyanide in an electric carbon tube, which produces iron carbide as a byproduct. Complexes Magnesium cyanide reacts with silver n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These positive ions can be exchanged for others in a contacting electrolyte solution. exchanged zeolites are particularly useful as solid acid catalysts. The term ''zeolite'' was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is ob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix ''octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicobalt Octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile. Synthesis, structure, properties Dicobalt octacarbonyl an orange-colored, pyrophoric solid. It is synthesised by the high pressure carbonylation of cobalt(II) salts: : The preparation is often carried out in the presence of cyanide, converting the cobalt(II) salt into a hexacyanocobaltate(II) complex that reacts with carbon monoxide to yield . Acidification produces cobalt tetracarbonyl hydride, , which degrades near room temperature to dicobalt octacarbonyl and hydrogen. It can also be prepared by heating cobalt metal t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalysis The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Polymer
A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions. It can also be described as a polymer whose repeat units are coordination complexes. Coordination polymers contain the subclass coordination networks that are coordination compounds extending, through repeating coordination entities, in 1 dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating coordination entities in 2 or 3 dimensions. A subclass of these are the metal-organic frameworks, or MOFs, that are coordination networks with organic ligands containing potential voids. Coordination polymers are relevant to many fields, having many potential applications. Coordination polymers can be classified in a number of ways accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Cyanide
Calcium cyanide is the inorganic compound with the chemical formula, formula Ca(CN)2. It is the calcium Salt (chemistry), salt derived from hydrocyanic acid. It is a white solid, although the pure material is rarely encountered. It hydrolyses readily (even in Moisture, moist air) to release hydrogen cyanide and is very toxic. Preparation Solutions of calcium cyanide can be prepared by treating calcium hydroxide with hydrogen cyanide. Solid calcium cyanide is produced commercially by heating calcium cyanamide with sodium chloride. The reaction is incomplete. The product is only of 50% purity, other components being sodium cyanide, calcium cyanamide, and carbon. Because of the carbon impurity, the solid is black, hence material is often called black cyanide. Reactivity At temperatures around 600 °C, calcium cyanide converts to calcium cyanamide:"Production of Hydrocyanic Acid" ''United States Patent Office.'' 1933.(accessed April 22, 2012). :Ca(CN)2 → CaCN2 + C It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanide
Sodium cyanide is a poisonous compound with the formula Na C N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. Production and chemical properties Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide: :HCN + NaOH → NaCN + H2O Worldwide production was estimated at 500,000 tons in the year 2006. Formerly it was prepared by the Castner process involving the reaction of sodium amide with carbon at elevated temperatures. : NaNH2 + C → NaCN + H2 The structure of solid NaCN is related to that of sodium chloride. The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. When treated with acid, it forms the toxic gas hydrogen cyanide: : NaCN + H+ → HCN + Na+ Because the salt is derived from a weak aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cyanide
Zinc cyanide is the inorganic compound with the formula Zn( CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds. Structure In Zn(CN)2, zinc adopts the tetrahedral coordination environment, all linked by bridging cyanide ligands. The structure consists of two "interpenetrating" structures (blue and red in the picture above). Such motifs are sometimes called "expanded diamondoid" structures. Some forms of SiO2 adopt a similar structure, wherein the tetrahedral Si centres are linked by oxides. The cyanide group shows head to tail disorder with any zinc atom having between one and four carbon neighbours, and the remaining being nitrogen atoms. It shows one of the largest negative coefficients of thermal expansion (exceeding the previous record holder, zirconium tungstate). Chemical properties Typical for an inorganic polymer, Zn(CN)2 is insoluble in most solvents. The solid disso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


8NoCo-Co.png)