|
Cis Effect
In inorganic chemistry, the cis effect is defined as the labilization (or destabilization) of CO ligands that are ''cis'' to other ligands. CO is a well-known strong pi-accepting ligand in organometallic chemistry that will labilize in the ''cis'' position when adjacent to ligands due to steric and electronic effects. The system most often studied for the ''cis'' effect is an octahedral complex where X is the ligand that will labilize a CO ligand ''cis'' to it. Unlike the ''trans'' effect, which is most often observed in 4-coordinate square planar complexes, the ''cis'' effect is observed in 6-coordinate octahedral transition metal complexes. It has been determined that ligands that are weak sigma donors and non-pi acceptors seem to have the strongest ''cis''-labilizing effects. Therefore, the ''cis'' effect has the opposite trend of the ''trans''-effect, which effectively labilizes ligands that are ''trans'' to strong pi accepting and sigma donating ligands. Electron counti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, although this term is barely heard. The acetyl group contains a methyl group () single-bonded to a carbonyl (). The carbonyl center of an acyl radical has one nonbonded electron with which it forms a chemical bond to the remainder ''R'' of the molecule. The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin). Acetylation In nature The introduction of an acetyl group into a molecule is called acetylation. In biological organisms, acetyl groups are commonly transferred from acetyl-CoA to other organic molecules. Acetyl-CoA is an intermediate both i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PPh3
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride Ion
The hydrogen anion, H−, is a negative ion of hydrogen, that is, a hydrogen atom that has captured an extra electron. The hydrogen anion is an important constituent of the atmosphere of stars, such as the Sun. In chemistry, this ion is called hydride. The ion has two electrons bound by the electromagnetic force to a nucleus containing one proton. The binding energy of H− equals the binding energy of an extra electron to a hydrogen atom, called electron affinity of hydrogen. It is measured to be or (see Electron affinity (data page)). The total ground state energy thus becomes . Occurrence The hydrogen anion is the dominant bound-free opacity source at visible and near-infrared wavelengths in the atmospheres of stars like the Sun and cooler; its importance was first noted in the 1930s. The ion absorbs photons with energies in the range 0.75–4.0 eV, which ranges from the infrared into the visible spectrum. Most of the electrons in these negative ions come from the i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
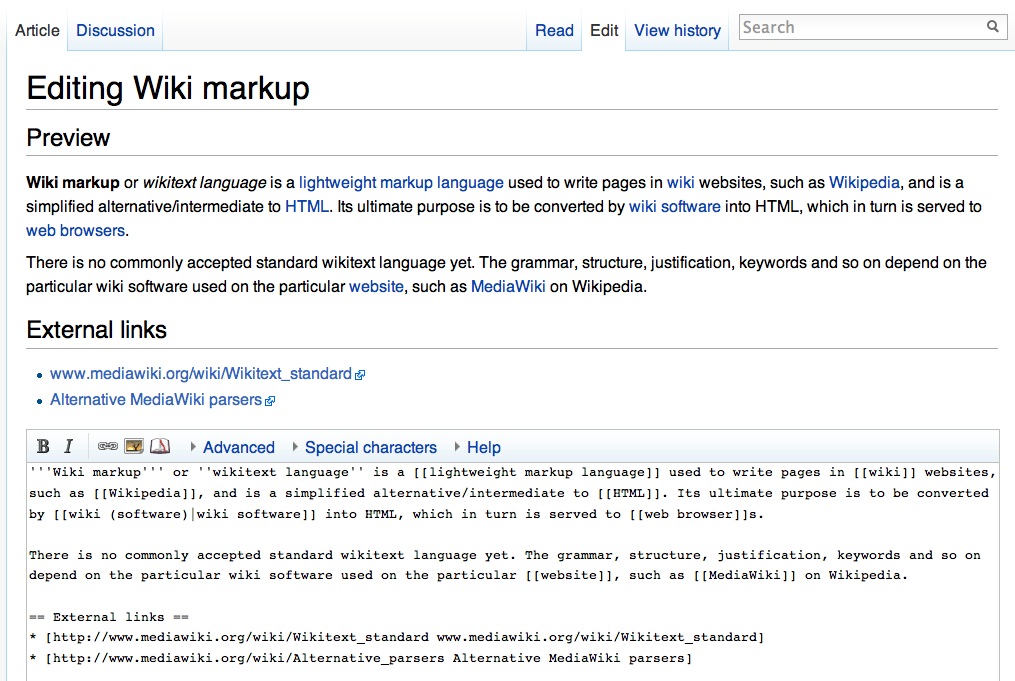
Wiki Figure
A wiki ( ) is an online hypertext publication collaboratively edited and managed by its own audience, using a web browser. A typical wiki contains multiple pages for the subjects or scope of the project, and could be either open to the public or limited to use within an organization for maintaining its internal knowledge base. Wikis are enabled by wiki software, otherwise known as wiki engines. A wiki engine, being a form of a content management system, differs from other web-based systems such as blog software, in that the content is created without any defined owner or leader, and wikis have little inherent structure, allowing structure to emerge according to the needs of the users. Wiki engines usually allow content to be written using a simplified markup language and sometimes edited with the help of a rich-text editor. There are dozens of different wiki engines in use, both standalone and part of other software, such as bug tracking systems. Some wiki engines are ope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN1 Reaction
The SN1 reaction is a substitution reaction in organic chemistry, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial rea ... is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using Steady state (chemistry), steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociative Substitution
In chemistry, dissociative substitution describes a reaction pathway by which compounds interchange ligands. The term is typically applied to coordination and organometallic complexes, but resembles the SN1 mechanism in organic chemistry. This pathway can be well described by the ''cis'' effect, or the labilization of CO ligands in the ''cis'' position. The opposite pathway is associative substitution, being analogous to SN2 pathway. Pathways that are intermediate between the pure dissociative and pure associative pathways are called interchange mechanisms. Complexes that undergo dissociative substitution are often coordinatively saturated and often have octahedral molecular geometry. The entropy of activation is characteristically positive for these reactions, which indicates that the disorder of the reacting system increases in the rate-determining step. Kinetics Dissociative pathways are characterized by a rate determining step that involves release of a ligand from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |