|
Circulene
A circulene is a macrocyclic arene in which a central polygon is surrounded and fused by benzenoids. Nomenclature within this class of molecules is based on the number of benzene rings surrounding the core, which is equivalent to the size of the central polygon. Examples which have been synthesized include irculene (corannulene), irculene (coronene), irculene, and 2irculene (kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas irculene is bowl-shaped and irculene is planar, irculene has a unique saddle-shaped structure (compare to cones and partial cones in calixarenes). The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix rather than a closed ring. Quadrannulene ( irculene) The simple irculene compound itself has not been synthesized, but a derivative, tetrabenzo irculene, also called quadrannulene, has. irculenes The isolation of the irculene derivative 2,5,6,9,10,13,14- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helicene
In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules. The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral, and optical features. Structure and properties The systematic naming for this class of compounds is based on the number of rings: 'n''elicene is the structure consisting of ''n'' rings. According to IUPAC, only structures where ''n'' is at least 5 are considered helicenes. Some specific compounds also have alternate or trivial names. As the number of rings increases, starting at four, the structure becomes non-planar, but instead the planes of consecutive rings tilt to prevent steric collisions. The resulting helix is chiral. For helicenes with six benzene units, a 360° turn is completed. In the helicene series the dihedral angles between the extremities increases going from e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kekulene
Kekulene is a polycyclic aromatic hydrocarbon which consists of 12 fused benzene rings arranged in a circle. It is therefore classified as a 2circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule. Geometry and electronic structure The nature of the π bonding within the molecule was long debated, as several distinctly different arrangements were possible. The two most significant proposals are the "Clar" configuration, consisting of six benzene-like (aromatic 6 π-electron) rings connected by bridging bonds and vinyl groups in non-aromatic rings, and the "Kékule" configuration, consisting of two concentric aromatic rings (18 π-electron inner, 30 π-electron outer) linked by radial single bonds. File:Kekulene (kekule conjugation).png, "Kékule" configuration: Two concentric aromatic rings File:Kekulene (linked benzenes).png, "Clar" config ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronene
Coronene (also known as superbenzene and cyclobenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is . It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene. The compound is of theoretical interest to organic chemists because of its aromaticity. It can be described by 20 resonance structures or by a set of three mobile Clar sextets. In the Clar sextet case, the most stable structure for coronene has only the three isolated outer sextets as fully aromatic although superaromaticity would still be possible when these sextets are able to migrate into the next ring. Occurrence and synthesis Coronene occurs naturally as the very rare mineral carpathite, which is characterized by flakes of pure coronene embedded in sedimentary rock. This mineral may be crea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20 H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is irculene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/ mol (42.7 kJ/mol) at −64 °C. Synthesis Several synthetic routes exist to corannulene. Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives. Corannulane was first isolated in 1966 by multistep organic synthesis. In 1971, the synthesis and properties of corannulane were reported. A flash vacuum pyrolysis method followed in 1991. One synthesis based on sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
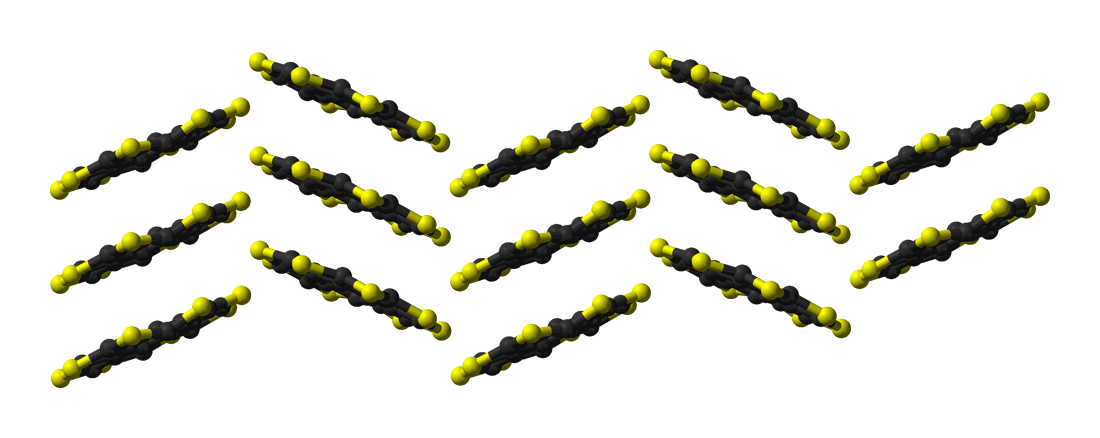
Sulflower
Sulflower (a portmanteau of sulfur and sunflower) is a stable heterocyclic octacirculene based on thiophene. Sulflower does not contain any hydrogen. With molecular formula (C2S)8 the compound is considered a type of carbon sulfide. The molecule is flat and together with the 9-membered homolog is at a local strain energy minimum. Its synthesis (a variation of the Ferrario reaction) is based on deprotonation of a tetrathiophene with lithium diisopropylamide followed by reaction with elemental sulfur to a sulfur-substituted intermediate followed by vacuum pyrolysis The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py .... The sulflower molecule has a planar structure with D8h symmetry, i.e., all eight sulfur atoms as well as the two faces of the molecule are undistinguishable. B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helix
A helix () is a shape like a corkscrew or spiral staircase. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined helices, and many proteins have helical substructures, known as alpha helices. The word ''helix'' comes from the Greek word ''ἕλιξ'', "twisted, curved". A "filled-in" helix – for example, a "spiral" (helical) ramp – is a surface called '' helicoid''. Properties and types The ''pitch'' of a helix is the height of one complete helix turn Turn may refer to: Arts and entertainment Dance and sports * Turn (dance and gymnastics), rotation of the body * Turn (swimming), reversing direction at the end of a pool * Turn (professional wrestling), a transition between face and heel * Turn, ..., measured parallel to the axis of the helix. A double helix consists of two (typically congruent) helices with the same axis, differing by a tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saddle
The saddle is a supportive structure for a rider of an animal, fastened to an animal's back by a girth. The most common type is equestrian. However, specialized saddles have been created for oxen, camels and other animals. It is not known precisely when riders first began to use some sort of padding or protection, but a blanket attached by some form of surcingle or girth was probably the first "saddle", followed later by more elaborate padded designs. The solid saddle tree was a later invention, and though early stirrup designs predated the invention of the solid tree, the paired stirrup, which attached to the tree, was the last element of the saddle to reach the basic form that is still used today. Today, modern saddles come in a wide variety of styles, each designed for a specific equestrianism discipline, and require careful fit to both the rider and the horse. Proper saddle care can extend the useful life of a saddle, often for decades. The saddle was a crucial step ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |