|
Chlorosulfonated Polyethylene
Hypalon is a chlorosulfonated polyethylene (CSPE) synthetic rubber (CSM) noted for its resistance to chemicals, temperature extremes, and ultraviolet light. It was a product of DuPont Performance Elastomers, a subsidiary of DuPont. Hypalon as it is now known in the marine industry today is a remarketed version of the old Hypalon using an additional layer of neoprene (cr) so the new chemical formulation is csm/cr. Chemical structure Polyethylene is treated with a mixture of chlorine and sulfur dioxide under UV-radiation. The product contains 20-40% chlorine. The polymer also contains a few percent chlorosulfonyl (ClSO2-) groups. These reactive groups allow for vulcanization, which strongly affects the physical durability of the products. An estimated 110,000 tons/y were produced in 1991. Discontinuance DuPont Performance Elastomers announced on May 7, 2009, that it intended to close its manufacturing plant in Beaumont, Texas Beaumont is a coastal city in the U.S. state of T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
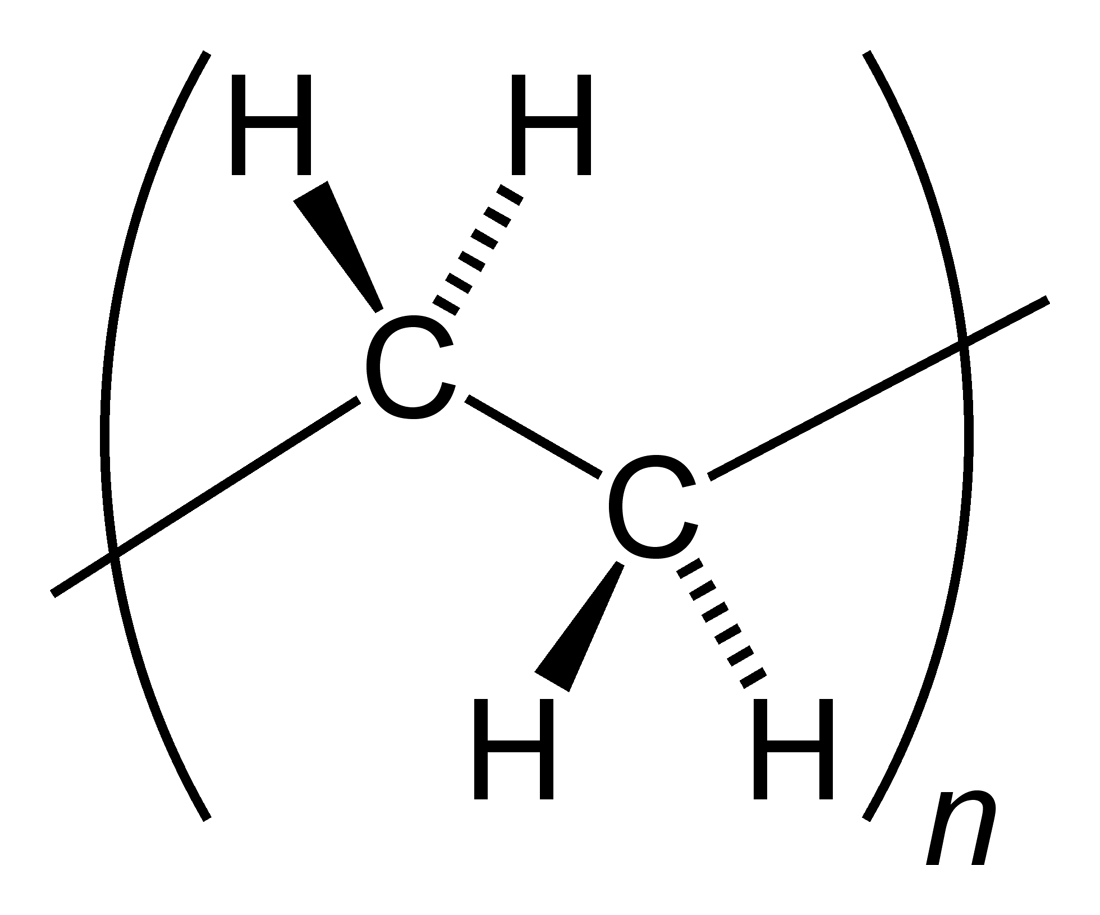
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About 32-million metric tons of rubbers are produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural rubber, has many uses in the automotive industry for tires, door and window profiles, seals such as O-rings and gaskets, hoses, belts, matting, and flooring. They offer a different range of physical and chemical properties, so can improve the reliability of a given product or application. Synthetic rubbers are superior to natural rubbers in two major respects, thermal stability and resistance to oils and related compounds. They are more resistant to oxidizing agents, such as oxygen and ozone which can reduce the life of products like tires. History of synthetic rubber The expanded use of bicycles, and particularly their pneumatic tires, starting in the 1890s, created increased demand for rubber. In 1909, a team he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet Light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surfaces with which it comes into conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in the development of Delaware and first arose as a major supplier of gunpowder. DuPont developed many polymers such as Vespel, neoprene, nylon, Corian, Polytetrafluoroethylene, Teflon, Mylar, Kapton, Kevlar, Zemdrain, M5 fiber, Nomex, Tyvek, Sorona, Artificial Leather#Corfam, Corfam and Lycra in the 20th century, and its scientists developed many chemicals, most notably Freon (chlorofluorocarbons), for the refrigerant industry. It also developed synthetic pigments and paints including ChromaFlair. In 2015, DuPont and the Dow Chemical Company agreed to a reorganization plan in which the two companies would merge and split into three. As a merged entity, DuPont simultaneously acquired Dow and renamed itself to DowDuPont on August 31, 2017, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides. Vulcanization can be defined as the curing of elastomers, with the terms 'vulcanization' and 'curing' sometimes used interchangeably in this context. It works by forming cross-links between sections of polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electrical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible. The word vulcanization is derived from Vulcan, the Roman god of fire and forge. History Rubber—latex� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beaumont, Texas
Beaumont is a coastal city in the U.S. state of Texas. It is the seat of government of Jefferson County, within the Beaumont– Port Arthur metropolitan statistical area, located in Southeast Texas on the Neches River about east of Houston (city center to city center). With a population of 115,282 at the 2020 census, Beaumont is the largest incorporated municipality by population near the Louisiana border. Its metropolitan area was the 10th largest in Texas in 2019, and 132nd in the United States. The city of Beaumont was founded in 1838. The pioneer settlement had an economy based on the development of lumber, farming, and port industries. In 1892, Joseph Eloi Broussard opened the first commercially successful rice mill in Texas, stimulating development of rice farming in the area; he also started an irrigation company (since 1933, established as the Lower Neches Valley Authority) to support rice culture. Rice became an important commodity crop in Texas and is now culti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastomers
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic polymer'', is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant ( E ≈ 3 M Pa) and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and dam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_(cropped).jpg)

