|
Chlorophyll B
} Chlorophyll ''b'' is a form of chlorophyll. Chlorophyll ''b'' helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll ''a'' in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light. In land plants, the light-harvesting antennae around photosystem II contain the majority of chlorophyll ''b''. Hence, in shade-adapted chloroplasts, which have an increased ratio of photosystem II to photosystem I, there is a higher ratio of chlorophyll ''b'' to chlorophyll ''a''. This is adaptive, as increasing chlorophyll ''b'' increases the range of wavelengths absorbed by the shade chloroplasts. Biosynthesis The Chlorophyll ''b'' biosynthetic pathway utilizes a variety of enzymes. In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme. The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthetic Pathway
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as ''catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate by cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytol
Phytol (florasol, phytosol) is an acyclic hydrogenated diterpene alcohol that can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1. In ruminants, the gut fermentation of ingested plant materials liberates phytol, a constituent of chlorophyll, which is then converted to phytanic acid and stored in fats. In shark liver it yields pristane. Human pathology Refsum disease (also known as adult Refsum disease) is an autosomal recessive disorder that results in the accumulation of toxic stores of phytanic acid in tissues and frequently manifests as a variable combination of peripheral polyneuropathy, cerebellar ataxia, retinitis pigmentosa, anosmia, and hearing loss. Although humans cannot derive phytanic acid from chlorophyll, they can convert free phytol into phytanic acid. Thus, patients with Refsum disease should limit their intake of phytanic acid and free phytol. The amount of free phytol in numerous food products has been reported. Roles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpene
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyllide
Chlorophyllide ''a'' and Chlorophyllide ''b'' are the biosynthetic precursors of chlorophyll ''a'' and chlorophyll ''b'' respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide ''a'' is also an intermediate in the biosynthesis of bacteriochlorophylls. Structures Chlorophyllide ''a'', is a carboxylic acid (R=H). In chlorophyllide ''b'', the methyl group at position 13 ( IUPAC numbering for chlorophyllide ''a'') and highlighted in the green box, is replaced with a formyl group. Biosynthesis steps up to formation of protoporphyrin IX In the early steps of the biosynthesis, which starts from glutamic acid, a tetrapyrrole is created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll Synthase
In enzymology, chlorophyll synthase () is an enzyme that catalyzes the chemical reaction :chlorophyllide a + phytyl diphosphate \rightleftharpoons chlorophyll a + diphosphate The two substrates of this enzyme are chlorophyllide ''a'' and phytyl diphosphate; its two products are chlorophyll ''a'' and diphosphate. The same enzyme can act on chlorophyllide ''b'' to form chlorophyll ''b''. Chlorophyllide a.svg, Chlorophyllide ''a'' Chlorophyll a structure.svg, Chlorophyll ''a'' This enzyme belongs to the family of transferases, specifically those transferring aryl or alkyl groups other than methyl groups. The systematic name of this enzyme class is chlorophyllide-a:phytyl-diphosphate phytyltransferase. This reaction is the final step of the complete biosynthetic pathway to chlorophylls from glutamic acid Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protoporphyrin
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not soluble in water. The name is often abbreviated as PPIX. Protoporphyrin IX contains a porphine core, a tetrapyrrole macrocycle with a marked aromatic character. Protoporphyrin IX is essentially planar, except for the N-H bonds that are bent out of the plane of the rings, in opposite (trans) directions. Nomenclature The general term protoporphyrin refers to porphine derivatives that have the outer hydrogen atoms in the four pyrrole rings replaced by other functional groups. The prefix proto often means 'first' in science nomenclature (such as carbon protoxide), hence Hans Fischer is thought to have coined the name protoporphyrin as the first class of porphyrins. Fischer described iron-deprived heme becoming the "proto-" porphyrin, particula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
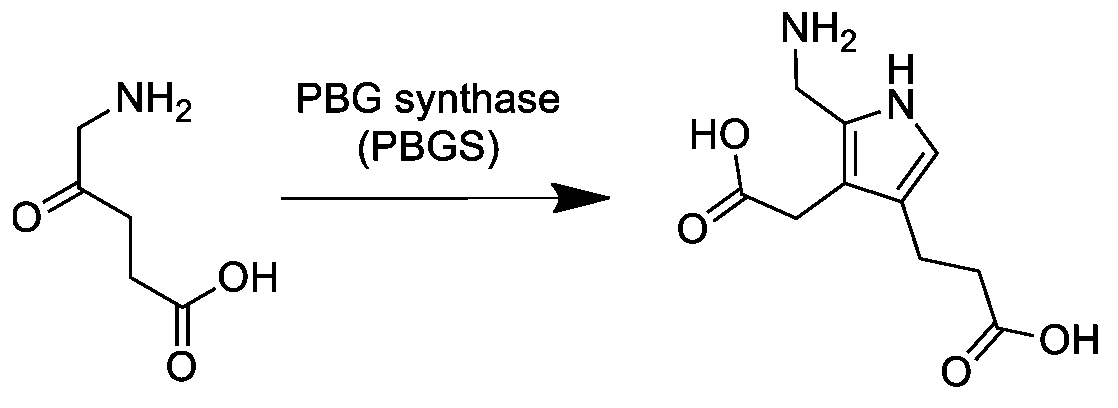
Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll. The structure of the molecule can be described as molecule of pyrrole with sidechains substituted for hydrogen atoms at positions 2, 3 and 4 in the ring (1 being the nitrogen atom); respectively, an aminomethyl group , an acetic acid (carboxymethyl) group , and a propionic acid (carboxyethyl) group . Biosynthesis In the first step of the porphyrin biosynthesis pathway, porphobilinogen is generated from aminolevulinate (ALA) by the enzyme ALA dehydratase. Metabolism In the typical porphyrin biosynthesis pathway, four molecules of porphobilinogen are concatenated by carbons 2 and 5 of the pyrrole ring (adjacent to the nitrogen atom) into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase. Pathologies Acute intermittent porphyr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Redox Reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-aminolevulinic Acid
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in plants. 5ALA is used in photodynamic detection and surgery of cancer.Wagnières, G.., Jichlinski, P., Lange, N., Kucera, P., Van den Bergh, H. (2014). Detection of Bladder Cancer by Fluorescence Cystoscopy: From Bench to Bedside - the Hexvix Story. Handbook of Photomedicine, 411-426. Medical uses As a precursor of a photosensitizer, 5ALA is also used as an add-on agent for photodynamic therapy. In contrast to larger photosensitizer molecules, it is predicted by computer simulations to be able to penetrate tumor cell membranes. Cancer diagnosis Photodynamic detection is the use of photosensitive drugs with a light source of the right wavelength for the detection of cancer, using fluorescence of the drug. 5ALA, or derivatives thereof, can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siroheme
Siroheme (or sirohaem) is a heme-like prosthetic group at the active sites of some enzymes to accomplish the six-electron reduction of sulfur and nitrogen. It is a cofactor at the active site of sulfite reductase, which plays a major role in sulfur assimilation pathway, converting sulfite into sulfide, which can be incorporated into the organic compound homocysteine. Biosynthesis Like all tetrapyrroles, the macrocyclic ligand in siroheme is derived from uroporphyrinogen III. This porphyrinogen is methylated at two adjacent pyrrole rings to give dihydrosirohydrochlorin, which is subsequently oxidized to give sirohydrochlorin Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which cat .... A ferrochelatase then inserts iron into the macrocycle to give siroheme. See also * Ferredoxin-nitrite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |