|
Chlorodiisopropylphosphine
Chlorodiisopropylphosphine is an organophosphorus compound with the formula [(CH3)2CH]2PCl. It is a colorless liquid that reacts with water and oxygen. The compound is used to prepare tertiary phosphines and phosphinite ligands. Synthesis and reactions The compound is prepared by treating phosphorus trichloride with the Grignard reagent isopropylmagnesium chloride: :PCl3 + 2 (CH3)2CHMgCl → [(CH3)2CH]2PCl + 2 MgCl2 Relative to the reaction of less hindered Grignard reagents with PCl3, this reaction affords a superior yield of the monochloro derivative. Chlorodiisopropylphosphine reacts with Grignard reagents and organolithium compounds to give phosphines: : [(CH3)2CH]2PCl + RM → [(CH3)2CH]2PR + MCl Chlorodiisopropylphosphine reacts with alcohols and phenols to give phosphinites, this reaction typically is conducted in the presence of a base: : [(CH3)2CH]2PCl + ROH → [(CH3)2CH]2POR + HCl Phosphinites are versatile ligands.for example: Pandarus, V., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropylmagnesium Chloride
Isopropylmagnesium chloride is an organometallic compound with the general formula (CH3)2HCMgCl. This highly flammable, colorless, and moisture sensitive material is the Grignard reagent derived from isopropyl chloride. It is commercially available, usually as a solution in tetrahydrofuran Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is .... This reagent is used to prepare Grignard reagents by transmetalation reactions as well as installing isopropyl groups. An illustrative generic reaction involves the generation of the Grignard reagent derived from bromo-3,5-bis(trifluoromethyl)benzene: :(CH3)2HCMgCl + (CF3)2C6H3Br → (CH3)2HCCl + (CF3)2C6H3MgBr Isopropylmagnesium chloride is also used to prepare other isopropyl compounds, such as chlorodiisopropylphosphine:{{cite jo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
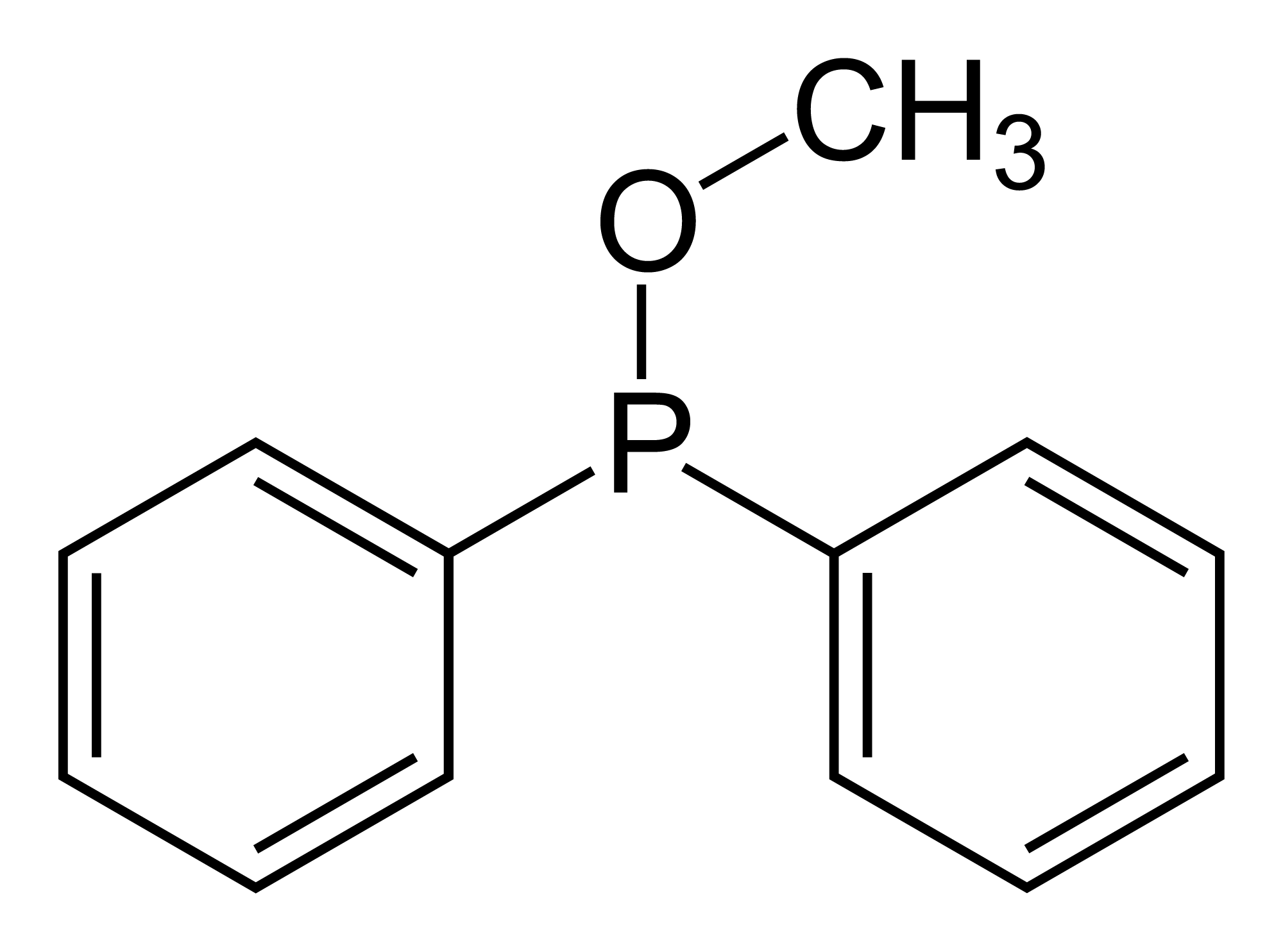
Phosphinite
In organic chemistry, phosphinites are organophosphorus compounds with the formula . They are used as ligands in homogeneous catalysis and coordination chemistry. Preparation Phosphinites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of chlorodiphenylphosphine with methanol and base gives methyl diphenylphosphinite: :ClPPh2 + CH3OH → CH3OPPh2 + HCl Although they are esters of phosphinous acids (R2POH), phosphinites are not made via such intermediates. Reactions Oxidation of phosphinites gives phosphinates: :2 P(OR)R2 + O2 → 2 OP(OR)R2 Phosphinites are ligands, giving derivatives similar to metal phosphine complexes. They are stronger pi-acceptors than typical phosphine ligands. References See also *Phosphine - PR3 * Phosphine oxide - OPR3 *Phosphonite - P(OR)2R *Phosphite - P(OR)3 * Phosphinate - OP(OR)R2 *Phosphonate - OP(OR)2R *Phosphate In chemistry, a phosphate is an anion, salt, functional group or ester derived f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphinite
In organic chemistry, phosphinites are organophosphorus compounds with the formula . They are used as ligands in homogeneous catalysis and coordination chemistry. Preparation Phosphinites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of chlorodiphenylphosphine with methanol and base gives methyl diphenylphosphinite: :ClPPh2 + CH3OH → CH3OPPh2 + HCl Although they are esters of phosphinous acids (R2POH), phosphinites are not made via such intermediates. Reactions Oxidation of phosphinites gives phosphinates: :2 P(OR)R2 + O2 → 2 OP(OR)R2 Phosphinites are ligands, giving derivatives similar to metal phosphine complexes. They are stronger pi-acceptors than typical phosphine ligands. References See also *Phosphine - PR3 * Phosphine oxide - OPR3 *Phosphonite - P(OR)2R *Phosphite - P(OR)3 * Phosphinate - OP(OR)R2 *Phosphonate - OP(OR)2R *Phosphate In chemistry, a phosphate is an anion, salt, functional group or ester derived f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphines
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisier, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


4-3D-balls.png)