|
Chlorites
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous acid. Compounds The free acid, chlorous acid HClO2, is the least stable oxoacid of chlorine and has only been observed as an aqueous solution at low concentrations. Since it cannot be concentrated, it is not a commercial product. The alkali metal and alkaline earth metal compounds are all colorless or pale yellow, with sodium chlorite (NaClO2) being the only commercially important chlorite. Heavy metal chlorites (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and ) are unstable and decompose explosively with heat or shock. Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. First, the explosively unstable gas chlorine dioxide, ClO2 is produced by reducing sodium chlorate with a suitable reducing agent such as methanol, hydrogen peroxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorous Acid
Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5): : 2 HClO2 → HClO + HClO3 Although the acid is difficult to obtain in pure substance, the conjugate base, chlorite, derived from this acid is stable. One example of a salt of this anion is the well-known sodium chlorite. This and related salts are sometimes used in the production of chlorine dioxide. Preparation HClO2 can be prepared through reaction of barium or lead chlorite and dilute sulfuric acid: :Ba(ClO2)2 + H2SO4 → BaSO4 + 2 HClO2 :Pb(ClO2)2 + H2SO4 → PbSO4 + 2 HClO2 Stability Chlorous acid is a powerful oxidizing agent, although its tendency to disproportionation counteracts its oxidizing potential. Chlorine is the only halogen to form an isolable acid of formula HXO2.Egon Wiberg, Arnol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
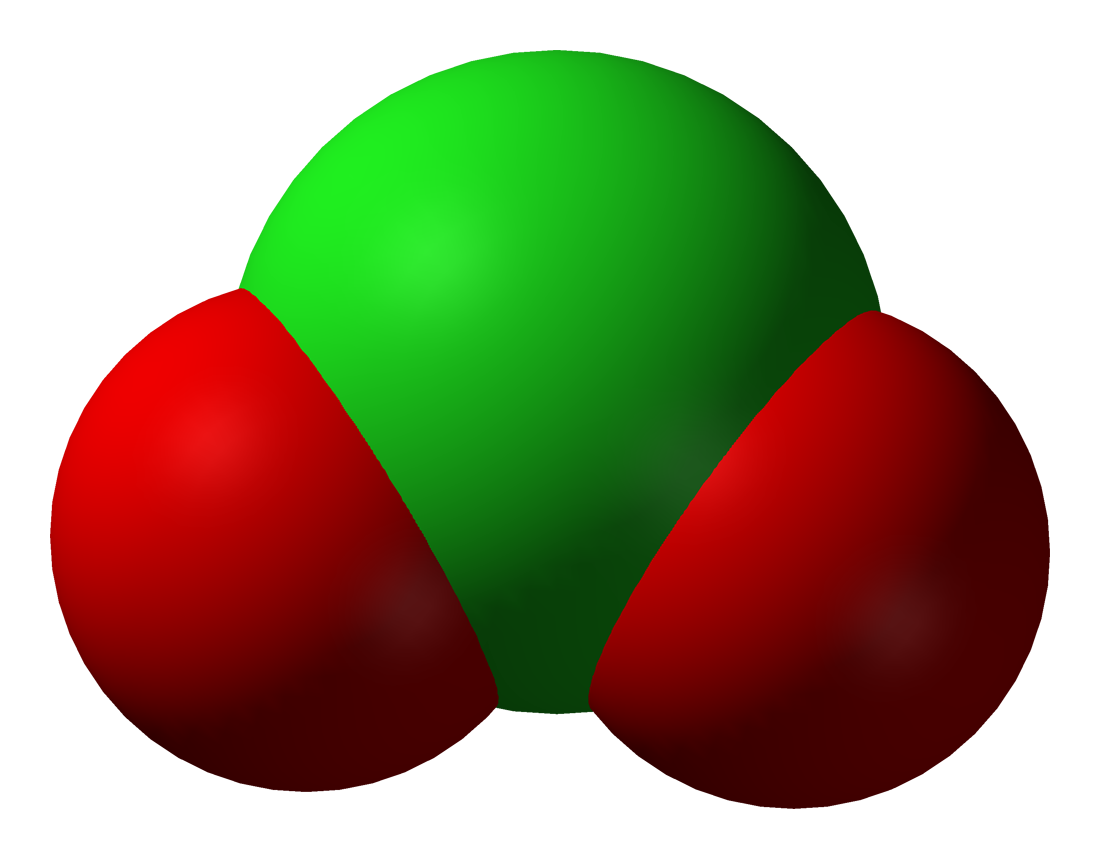
Halite (oxyanion)
A halite, also known as a halogenite, is an oxyanion containing a halogen The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ... in a III oxidation state. It is the conjugate base of a halous acid. The known halites are chlorite, bromite, and iodite. Uses Halites can be used to generate the respective halogen dioxides via a one-electron oxidation: :Sodium chlorite, 5 NaClO2 + Hydrochloric acid, 4 HCl → Sodium chloride, 5 NaCl + Chlorine dioxide, 4 + Properties of water, 2 H2O :Bromite, + Bromic acid, HBrO3 + H+ → Bromine dioxide, 2 + Properties of water, H2O This reaction in particular is used in bleach to generate chlorine dioxide. Stability Chlorites tend to decompose rapidly, some even explosively, upon heating. A few bromites have been isolated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chlorite
Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant. Use The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection of municipal water treatment plants after conversion to chlorine dioxide. An advantage in this application, as compared to the more commonly used chlorine, is that trihalomethanes (such as chloroform) are not produced from organic contaminants. Chlorine dioxide generated from sodium chlorite is approved by FDA under some conditions for disinfecting water used to wash fruits, vegetables, and poultry. Sodium chlorite, NaClO2, sometimes in combination with zinc chloride, also finds application as a component in therapeutic rinses, mouthwashes, toothpastes and gels, mouth sprays, as preservative in eye drops, and in contact lens cleaning solution under the trade name Purite. It is also used for sanitizing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorous Acid
Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5): : 2 HClO2 → HClO + HClO3 Although the acid is difficult to obtain in pure substance, the conjugate base, chlorite, derived from this acid is stable. One example of a salt of this anion is the well-known sodium chlorite. This and related salts are sometimes used in the production of chlorine dioxide. Preparation HClO2 can be prepared through reaction of barium or lead chlorite and dilute sulfuric acid: :Ba(ClO2)2 + H2SO4 → BaSO4 + 2 HClO2 :Pb(ClO2)2 + H2SO4 → PbSO4 + 2 HClO2 Stability Chlorous acid is a powerful oxidizing agent, although its tendency to disproportionation counteracts its oxidizing potential. Chlorine is the only halogen to form an isolable acid of formula HXO2.Egon Wiberg, Arnol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful effects on human health. Perchlorate ions are somewhat toxic to the thyroid gland. Most perchlorates are colorless solids that are soluble in water. Four perchlorates are of primary commercial interest: ammonium perchlorate , perchloric acid , potassium perchlorate and sodium perchlorate . Perchlorate is the anion resulting from the dissociation of perchloric acid and its salts upon their dissolution in water. Many perchlorate salts are soluble in non-aqueous solutions. Production Perchlorate salts are produced industrially by the oxidation of aqueous solutions of sodium chlorate by electrolysis. This method is used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyanion
An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound . The structures of condensed oxyanions can be rationalized in terms of AO''n'' polyhedral units with sharing of corners or edges between polyhedra. The oxyanions (specifically, phosphate and polyphosphate esters) adenosine monophosphate ( AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology. Monomeric oxyanions The formula of monomeric oxyanions, , is dictated by the oxidation state of the element A and its position in the periodic table. Elements of the first row are limited to a maximum coordination number of 4. However, none of the first row elements has a monomeric oxyanion with that coordination number. Instead, ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine Oxide
Chlorine and oxygen can bond in many ways: * chlorine monoxide, , chlorine (II) oxide * chlorine peroxide, , dimer of chlorine (II) oxide *chlorine dioxide, , chlorine (IV) oxide * chloroperoxyl, *chlorine trioxide, ClO3, chlorine (VI) oxide *dichlorine monoxide, Cl2O, chlorine (I) oxide *Three dichlorine dioxides: **ClO dimer, Cl2O2, chlorine (I) peroxide **chloryl chloride, ClO2Cl, chlorine (0,IV) oxide ** chlorine chlorite, ClOClO, chlorine (I,III) oxide * dichlorine trioxide, Cl2O3, chlorine (I,V) oxide *dichlorine tetroxide, also known as chlorine perchlorate, ClOClO3, chlorine (I,VII) oxide *dichlorine pentoxide, Cl2O5, is hypothetical *dichlorine hexoxide, chloryl perchlorate, Cl2O6, chlorine (V,VII) oxide *dichlorine heptoxide, Cl2O7, chlorine (VII) oxide *chlorine tetroxide, * chlorine (VII) oxide peroxide, (OClO3)2 Several ions are also chlorine oxides: *chloryl, * perchloryl, * hypochlorite, ClO− * chlorite, ClO2− * chlorate, ClO3− *perchlorate, ClO4− See a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of bleaching powder, swimming pool "chlorine"). The Cl-O distance in ClO− is 1.69 Å. The name can also refer to esters of hypochlorous acid, namely organic compounds with a ClO– moiety (chemistry), group covalent bond, covalently bound to the rest of the molecule. The principal example is tert-butyl hypochlorite, which is a useful chlorinating agent. Most hypochlorite salts are handled as aqueous solutions. Their primary applications are as bleaching, disinfection, and water treatment agents. They are also used in chemistry for Halogenation, chlorination and oxidation reactions. Reactions Acid reaction Acidification of hypochlorites generates hypochlorous acid, which exists in an equilibrium with chlorine. A high pH drives the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half Cell
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a potential difference between the electrode and the electrolyte. The typical anode reaction involves a metal atom in the electrode being dissolved and transported as a positive ion across the double layer, causing the electrolyte to acquire a net positive charge while the electrode acquires a net negative charge. The growing potential difference creates an intense electric field within the double layer, and the potential rises in value until the field halts the net charge-pumping reactions. This self-limiting action occurs almost instantly in an isolated half-cell; in applications two dissimilar half-cells are appropriately connected to constitute a Galvanic cell. A standa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |