|
Chiral Lewis Acid
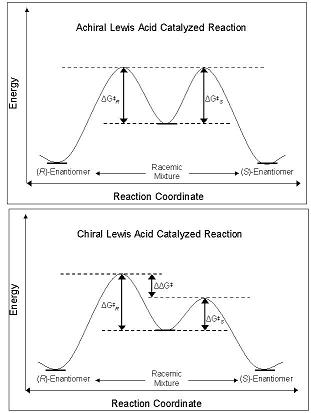
Chiral Lewis acids (CLAs) are a type of Lewis acid catalyst. These acids affect the chirality of the substrate as they react with it. In such reactions, synthesis favors the formation of a specific enantiomer or diastereomer. The method is an enantioselective asymmetric synthesis reaction. Since they affect chirality, they produce optically active products from optically inactive or mixed starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is formally known as asymmetric induction. In this kind of Lewis acid, the electron-accepting atom is typically a metal, such as indium, zinc, lithium, aluminium, titanium, or boron. The chiral-altering ligands employed for synthesizing these acids often have multiple Lewis basic sites (often a diol or a dinitrogen structure) that allow the formation of a ring structure involving the metal atom. Achiral Lewis acids have been used for decades to promote the synthesis of racemic mixtures in my ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic Mixtures
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harry Stone Mosher
Harry Stone Mosher (August 31, 1915 – March 2, 2001) was an American chemist and the discoverer of Mosher's acid. Early life Mosher attended Willamette University in Salem, Oregon, where he received a bachelor's degree in chemistry in 1937. He went on to Oregon State University, where he earned a master's degree in 1938. He then returned to Willamette to teach for one year. In 1939, he continued his graduate work at Pennsylvania State University under the mentorship of Frank C. Whitmore, a renowned organic chemist. In 1942, Mosher completed his PhD in organic chemistry. He remained at Pennsylvania State as an assistant professor, supervising research on synthetic anti-malarial drugs for the National Research Council and the production of DDT with the War Production Board. In 1944, Mosher married Carol Walker, a fellow chemistry graduate student at the university. Three years later, Mosher accepted an assistant professorship at Stanford University in the Department o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomeric Excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a single completely pure enantiomer has an ee of 100%. A sample with 70% of one enantiomer and 30% of the other has an ee of 40% (70% − 30%). Definition Enantiomeric excess is defined as the absolute difference between the mole fraction of each enantiomer: :\ ee = , F_R - F_S, where :\ F_R + F_S = 1 In practice, it is most often expressed as a percent enantiomeric excess. The enantiomeric excess can be determined in another way if we know the amount of each enantiomer produced. If one knows the moles of each enantiomer produced then: Enantiomeric excess is used as one of the indicators of the success of an asymmetric synthesis. For mixtures of diastereomers, there are analogous definitions and uses for diastereomeric exces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
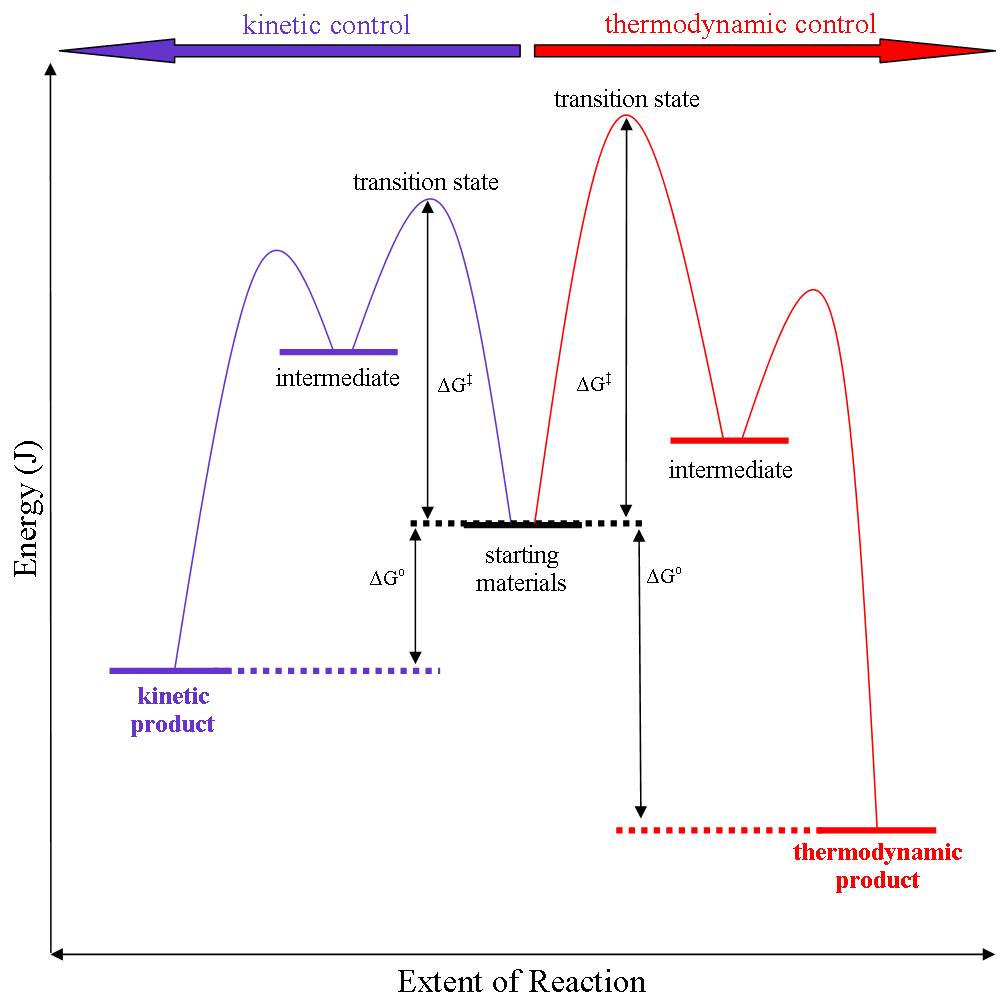
Kinetic Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state. Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecular dynamics simulations, a reaction coordinate is called collective variable. These coordinates can sometimes represent a real coordinate system (such as bond length, bond angle...), although, for more complex reactions especially, this can be difficult (and non geometric parameters are used, e.g., bond order). Reaction coordinate is distinct from extent of reaction, a different parameter of reaction progress, which is a measure of the ''composition'' of the reaction system. (Free) energy is often plotted against reaction coordinate(s) to demonstrate in some schematic form the potential energy profile (an intersection of a potential energy surface) associated with the reaction. In the formalism of transition-state theory the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joule per mole, joules per mole (J/mol), joule per mole, kilojoules per mole (kJ/mol) or kilocalorie per mole, kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral And Achiral Lewis Acid Catalyzed Reactions
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, ''enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
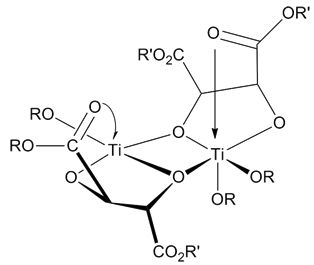
Sharpless Epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is ''tert''-butyl hydroperoxide. The method relies on a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate. 2,3-Epoxyalcohols can be converted into diols, aminoalcohols, and ethers. The reactants for the Sharpless epoxidation are commercially available and relatively inexpensive. K. Barry Sharpless was awarded the 2001 Nobel Prize in Chemistry for this and related work on asymmetric oxidations. The prize was shared with William S. Knowles and Ryōji Noyori. Catalyst 5–10 mol% of the catalyst is typical. The presence of 3Å molecular sieves (3Å MS) is necessary. The structure of the catalyst is uncertain although it is thought to be a dimer of []. Selectivity The epoxidation of allylic alcohols is a well-utilized conversion in fine chemical synthesis. The chirality of the produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocyanation
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon. Hydrocyanation of unactivated alkenes Industrially, hydrocyanation is commonly performed on alkenes catalyzed by nickel complexes of phosphite () ligands. A general reaction is shown:Piet W.N.M. van Leeuwen "Homogeneous Catalysis: Understanding the Art", 2004, Wiley-VCH, Weinheim. :RCH=CH2 + HCN -> RCH2-CH2-CN Stoichiometry and mechanism The reaction involves the addition of and cyanide () to the substrate. Usually the substrate is an alkene and the product is a nitrile. The reaction proceeds via the oxidative addition of HCN to a low-valent metal complex to give a hydrido cyanide complex. Subsequent binding of the alkene gives the intermediate , which then undergoes migratory insertion to give an alkylmetal cyani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |