|
Chemical Matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic particles, and in everyday as well as scientific usage, "matter" generally includes atoms and anything made up of them, and any particles (or combination of particles) that act as if they have both rest mass and volume. However it does not include massless particles such as photons, or other energy phenomena or waves such as light or heat. Matter exists in various states (also known as phases). These include classical everyday phases such as solid, liquid, and gas – for example water exists as ice, liquid water, and gaseous steam – but other states are possible, including plasma, Bose–Einstein condensates, fermionic condensates, and quark–gluon plasma. Usually atoms can be imagined as a nucleus of protons and neutrons, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Discharge Tube
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice ( crystalline solids, which include metals and ordinary ice), or irregularly (an amorphous solid such as common window glass). Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed. The branch of physics that deals with solids is called solid-state physics, and is the main branch of condensed matter physics (which also includes liquids). Materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary ( macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the Atomic nucleus, nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each chemical element, element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek language, G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brookhaven National Laboratory
Brookhaven National Laboratory (BNL) is a United States Department of Energy national laboratory located in Upton, Long Island, and was formally established in 1947 at the site of Camp Upton, a former U.S. Army base and Japanese internment camp. Its name stems from its location within the Town of Brookhaven, approximately 60 miles east of New York City. It is managed by Stony Brook University and Battelle Memorial Institute. Research at BNL includes nuclear and high energy physics, energy science and technology, environmental and bioscience, nanoscience, and national security. The 5,300 acre campus contains several large research facilities, including the Relativistic Heavy Ion Collider and National Synchrotron Light Source II. Seven Nobel Prizes have been awarded for work conducted at Brookhaven Lab. Overview BNL is staffed by approximately 2,750 scientists, engineers, technicians, and support personnel, and hosts 4,000 guest investigators every year. The laboratory has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quark–gluon Plasma
Quark–gluon plasma (QGP) or quark soup is an interacting localized assembly of quarks and gluons at thermal (local kinetic) and (close to) chemical (abundance) equilibrium. The word ''plasma'' signals that free color charges are allowed. In a 1987 summary, Léon van Hove pointed out the equivalence of the three terms: quark gluon plasma, quark matter and a new state of matter. Since the temperature is above the Hagedorn temperature—and thus above the scale of light u,d-quark mass—the pressure exhibits the relativistic Stefan-Boltzmann format governed by temperature to the fourth power ( T^) and many practically massless quark and gluon constituents. It can be said that QGP emerges to be the new phase of strongly interacting matter which manifests its physical properties in terms of nearly free dynamics of practically massless gluons and quarks. Both quarks and gluons must be present in conditions near chemical (yield) equilibrium with their colour charge ''open'' for a new ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermionic Condensate
A fermionic condensate or Fermi–Dirac condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar conditions. The earliest recognized fermionic condensate described the state of electrons in a superconductor; the physics of other examples including recent work with fermionic atoms is analogous. The first atomic fermionic condensate was created by a team led by Deborah S. Jin using potassium-40 atoms at the University of Colorado Boulder in 2003. Background Superfluidity Fermionic condensates are attained at lower temperatures than Bose–Einstein condensates. Fermionic condensates are a type of superfluid. As the name suggests, a superfluid possesses fluid properties similar to those possessed by ordinary liquids and gases, such as the lack of a definite shape and the ability to flow in response to applied forces. However, superfluids p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
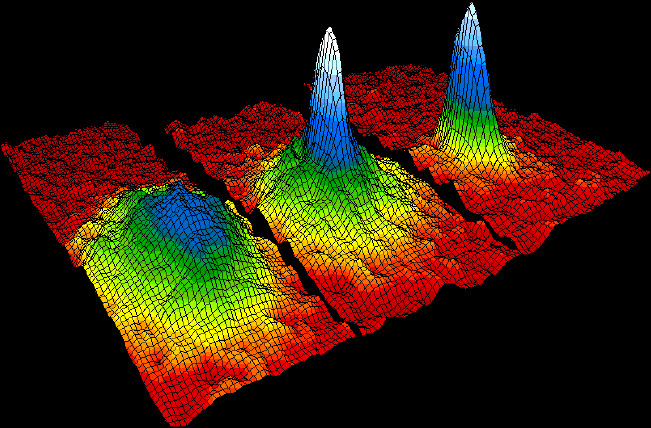
Bose–Einstein Condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.67 °F). Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which point microscopic quantum mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. A BEC is formed by cooling a gas of extremely low density (about 100,000 times less dense than normal air) to ultra-low temperatures. This state was first predicted, generally, in 1924–1925 by Albert Einstein following and crediting a pioneering paper by Satyendra Nath Bose on the new field now known as quantum statistics. In 1995, the Bose-Einstein condensate was created by Eric Cornell and Carl Wieman of the University of Colorado at Boulder using rubidium atoms; later that year, Wolfgang Ketterle of MIT prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma (physics)
Plasma ()πλάσμα , Henry George Liddell, Robert Scott, ''A Greek English Lexicon'', on Perseus is one of the four fundamental states of matter. It contains a significant portion of charged particles – ions and/or s. The presence of these charged particles is what primarily sets plasma apart from the other fundamental states of matter. It is the most abundant form of [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |