|
Charge Transport Mechanisms
Charge transport mechanisms are theoretical models that aim to quantitatively describe the electric current flow through a given medium. Theory Crystalline solids and molecular solids are two opposite extreme cases of materials that exhibit substantially different transport mechanisms. While in atomic solids transport is ''intra''-molecular, also known as band transport, in molecular solids the transport is ''inter''-molecular, also known as hopping transport. The two different mechanisms result in different charge mobilities. In disordered solids, disordered potentials result in weak localization effects (traps), which reduce the mean free path, and hence the mobility, of mobile charges. Carrier recombination also decreases mobility. Starting with Ohm's law and using the definition of conductivity, it is possible to derive the following common expression for current as a function of carrier mobility μ and applied electric field E: :I=GV=\sigma\fracV=\sigma AE=en\mu AE The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or holes. In an electrolyte the charge carriers are ions, while in plasma, an ionized gas, they are ions and electrons. The SI unit of electric current is the ampere, or ''amp'', which is the flow of electric charge across a surface at the rate of one coulomb per second. The ampere (symbol: A) is an SI base unit. Electric current is measured using a device called an ammeter. Electric currents create magnetic fields, which are used in motors, generators, inductors, and transformers. In ordinary con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
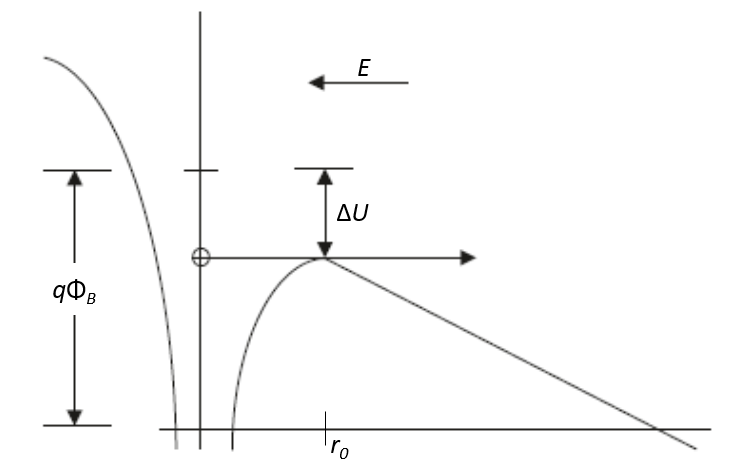
Fowler-Nordheim Equation
Field electron emission, also known as field emission (FE) and electron field emission, is emission of electrons induced by an electrostatic field. The most common context is field emission from a solid surface into a vacuum. However, field emission can take place from solid or liquid surfaces, into a vacuum, a fluid (e.g. air), or any non-conducting or weakly conducting dielectric. The field-induced promotion of electrons from the valence to conduction band of semiconductors (the Zener effect) can also be regarded as a form of field emission. The terminology is historical because related phenomena of surface photoeffect, thermionic emission (or Richardson–Dushman effect) and "cold electronic emission", i.e. the emission of electrons in strong static (or quasi-static) electric fields, were discovered and studied independently from the 1880s to 1930s. When field emission is used without qualifiers it typically means "cold emission". Field emission in pure metals occurs in high el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Resistance And Conductance
The electrical resistance of an object is a measure of its opposition to the flow of electric current. Its reciprocal quantity is , measuring the ease with which an electric current passes. Electrical resistance shares some conceptual parallels with mechanical friction. The SI unit of electrical resistance is the ohm (), while electrical conductance is measured in siemens (S) (formerly called the 'mho' and then represented by ). The resistance of an object depends in large part on the material it is made of. Objects made of electrical insulators like rubber tend to have very high resistance and low conductance, while objects made of electrical conductors like metals tend to have very low resistance and high conductance. This relationship is quantified by resistivity or conductivity. The nature of a material is not the only factor in resistance and conductance, however; it also depends on the size and shape of an object because these properties are extensive rather than inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons. Electrochemical processes are ET reaction. ET reactions are relevant to photosynthesis and respiration. ET reactions commonly involve transition metal complexes, In organic chemistry ET is a step in some commercial polymerization reactions. It is foundational to photoredox catalysis. Classes of electron transfer Inner-sphere electron transfer In inner-sphere ET, the two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous exa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann's Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poole–Frenkel Effect
In solid-state physics, the Poole–Frenkel effect (also known as Frenkel-Poole emissionSze, S. M., ''Physics of Semiconductor Devices'', 2nd edition, Section 4.3.4.) is a model describing the mechanism of trap-assisted electron transport in an electrical insulator. It is named after Yakov Frenkel, who published on it in 1938, extending the theory previously developed by H. H. Poole. Electrons can move slowly through an insulator by the following process. The electrons are generally trapped in localized states (loosely speaking, they are "stuck" to a single atom, and not free to move around the crystal). Occasionally, random thermal fluctuations will give an electron enough energy to leave its localized state, and move to the conduction band. Once there, the electron can move through the crystal, for a brief amount of time, before relaxing into another localized state (in other words, "sticking" to a different atom). The Poole–Frenkel effect describes how, in a large electric field ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arrhenius Equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 1884 that the van 't Hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. This equation has a vast and important application in determining the rate of chemical reactions and for calculation of energy of activation. Arrhenius provided a physical justification and interpretation for the formula. Laidler, K. J. (1987) ''Chemical Kinetics'', Third Edition, Harper & Row, p. 42 Currently, it is best seen as an empirical relationship.Kenneth Connors, Chemical Kinetics, 1990, VCH Publishers It can be used to model the temperature variation of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally-induced processes/r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
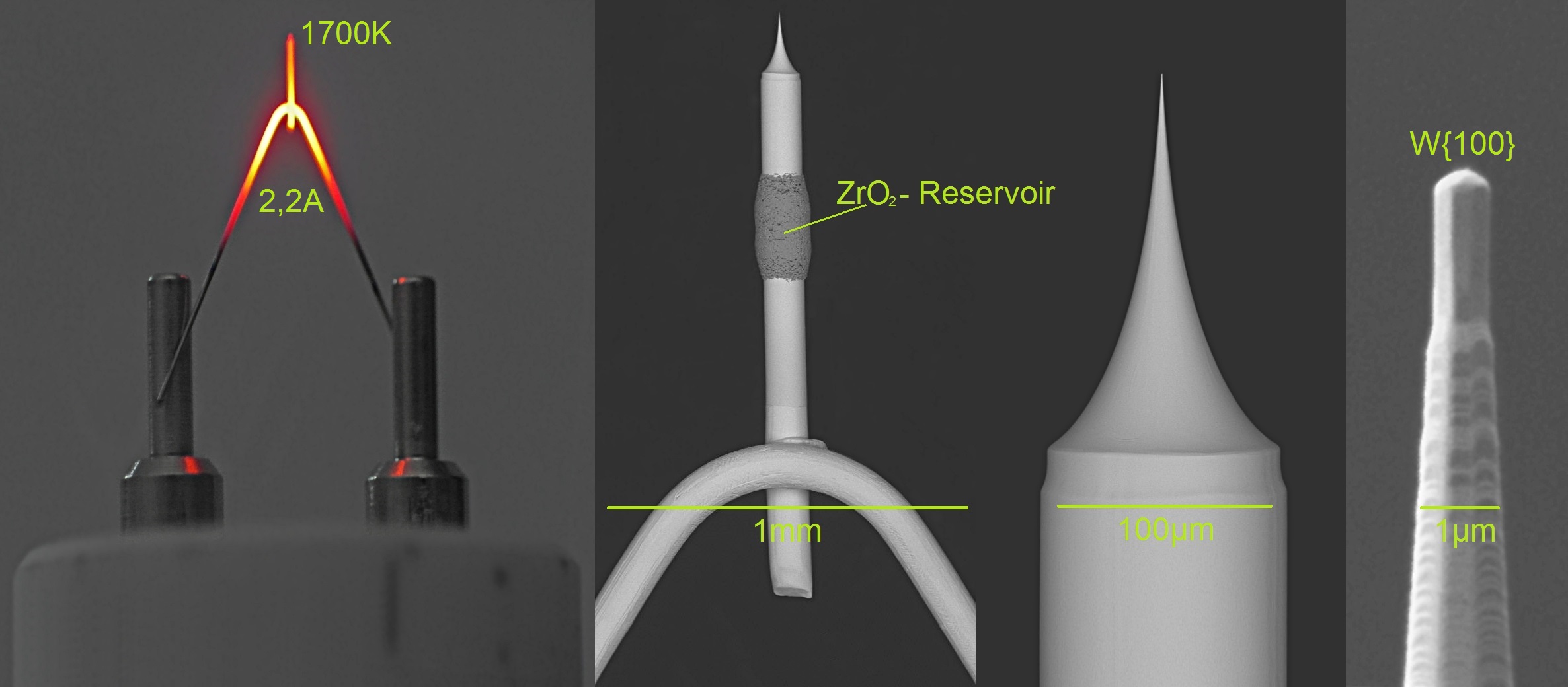
Thermionic Emission
Thermionic emission is the liberation of electrons from an electrode by virtue of its temperature (releasing of energy supplied by heat). This occurs because the thermal energy given to the charge carrier overcomes the work function of the material. The charge carriers can be electrons or ions, and in older literature are sometimes referred to as thermions. After emission, a charge that is equal in magnitude and opposite in sign to the total charge emitted is initially left behind in the emitting region. But if the emitter is connected to a battery, the charge left behind is neutralized by charge supplied by the battery as the emitted charge carriers move away from the emitter, and finally the emitter will be in the same state as it was before emission. The classical example of thermionic emission is that of electrons from a hot cathode into a vacuum (also known as thermal electron emission or the Edison effect) in a vacuum tube. The hot cathode can be a metal filament, a coated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Field Electron Emission
Field electron emission, also known as field emission (FE) and electron field emission, is emission of electrons induced by an electrostatic field. The most common context is field emission from a solid surface into a vacuum. However, field emission can take place from solid or liquid surfaces, into a vacuum, a fluid (e.g. air), or any non-conducting or weakly conducting dielectric. The field-induced promotion of electrons from the valence (chemistry), valence to conduction band of semiconductors (the Zener effect) can also be regarded as a form of field emission. The terminology is historical because related phenomena of surface photoeffect, thermionic emission (or Richardson–Dushman effect) and "cold electronic emission", i.e. the emission of electrons in strong static (or quasi-static) electric fields, were discovered and studied independently from the 1880s to 1930s. When field emission is used without qualifiers it typically means "cold emission". Field emission in pure metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Break Junction
A break junction is an electronic device which consists of two metal wires separated by a very thin gap, on the order of the inter-atomic spacing (less than a nanometer). This can be done by physically pulling the wires apart or through chemical etching or electromigration. As the wire breaks, the separation between the electrodes can be indirectly controlled by monitoring the electrical resistance of the junction. After the gap is formed, its width can often be controlled by bending the substrate that the metal contacts lie on. The gap can be controlled to a precision of picometers. A typical conductance versus time trace during the breaking process (conductance is simply current divided by applied voltage bias) shows two regimes. First is a regime where the break junction comprises a quantum point contact. In this regime conductance decreases in steps equal to the conductance quantum G_Q=2e^2/h which is expressed through the electron charge (−''e'') and Planck's constant h. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |