|
Cerium(III) Oxide
Cerium(III) oxide, also known as cerium oxide, cerium trioxide, cerium sesquioxide, cerous oxide or dicerium trioxide, is an oxide of the rare-earth metal cerium. It has chemical formula and is gold-yellow in color. According to X-ray crystallography, the Ce(III) ions are seven-coordinate, a motif typical for other trivalent lanthanide oxides. Applications Cerium oxide is of commercial interest as a catalyst for oxidation of carbon monoxide and reduction of . These applications exploit the facility of the Ce(III)/Ce(IV) redox couple. It is used in catalytic converters ("three-way catalytic converter") for the minimisation of CO emissions in the exhaust gases from motor vehicles. When there is a shortage of oxygen, cerium(IV) oxide oxidizes carbon monoxide to the benign dioxide: : When oxygen is in surplus, the process is reversed and cerium(III) oxide is oxidized to cerium(IV) oxide: : Cerium oxide-based catalysts have been intensively investigated for selective catalytic red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Oxide
Vanadium oxide mainly refers to: * Vanadium(II) oxide (vanadium monoxide), VO * Vanadium(III) oxide (vanadium sesquioxide ''or'' trioxide), V2O3 * Vanadium(IV) oxide (vanadium dioxide), VO2 * Vanadium(V) oxide (vanadium pentoxide), V2O5 Various other distinct phases include: * Phases with the general formula VnO2n+1 exist between V2O5 and VO2. Examples of these phases include V3O7, V4O9 and V6O13. * Phases with the general formula VnO2n−1 exist between VO2 and V2O3. Called Magnéli phases foArne Magnéli they are examples of crystallographic shear compounds based on the rutile structure. Examples of Magnéli phases include V4O7, V5O9, V6O11, V7O13 and V8O15. * V3O5 appears as the mineral oxyvanite. Many vanadium-oxygen phases are non-stoichiometric Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having chemical element, elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were fired clay bricks used for building house walls and other structures. Other pottery objects such as pots, vessels, vases and figurines were made from clay, either by itself or mixed with other materials like silica, hardened by sintering in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial, and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as semiconductors. The word '' ceramic'' comes from the Ancient Greek word (), meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(II) Oxide
Tin(II) oxide (stannous oxide) is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form. Preparation and reactions Blue-black SnO can be produced by heating the tin(II) oxide hydrate, (''x'' < 1) precipitated when a tin(II) salt is reacted with an alkali hydroxide such as NaOH.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier Metastable, red SnO can be prepared by gentle heating of the precipitate produced by the action of aqueous ammonia on a tin(II) salt. SnO may be prepared as a pure substance in the laboratory, by controlled heating of tin(II) oxalate ( stannous oxalate) in the absence of air or under a CO2 atmosphere. This method is als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
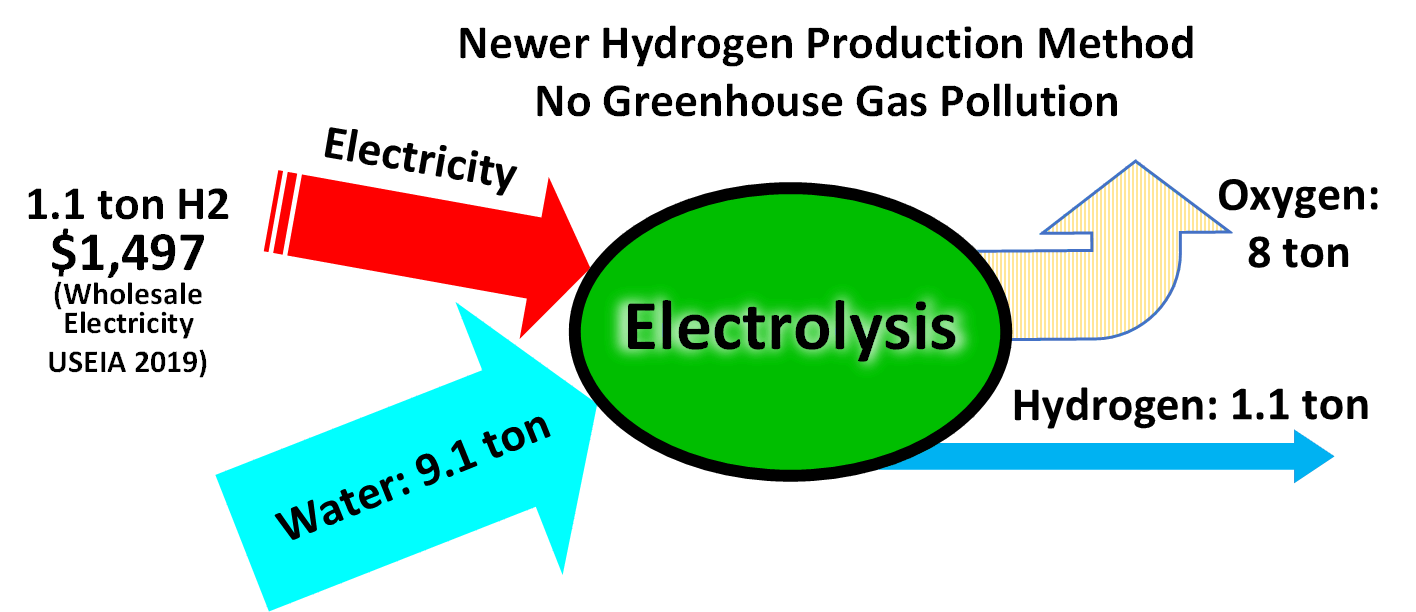
Water Splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen: Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy. A version of water splitting occurs in photosynthesis, but hydrogen is not produced. The reverse of water splitting is the basis of the hydrogen fuel cell. Water splitting using solar radiation has not been commercialized. Electrolysis Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen (H2): Production of hydrogen from water is energy intensive. Usually, the electricity consumed is more valuable than the hydrogen produced, so this method has not been widely used. In contrast with low-temperature electrolysis, high-temperature electrolysis (HTE) of water converts more of the initial heat energy into chemical energy (hydrogen), potentially doubling efficiency to about 50%. Because some of the energy in HTE is supplied in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermochemistry
Thermochemistry is the study of the heat energy which is associated with chemical reactions and/or phase changes such as melting and boiling. A reaction may release or absorb energy, and a phase change may do the same. Thermochemistry focuses on the energy exchange between a system and its surroundings in the form of heat. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable. Endothermic reactions absorb heat, while exothermic reactions release heat. Thermochemistry coalesces the concepts of thermodynamics with the concept of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity, heat of combustion, heat of formation, enthalpy, entropy, and free energy. Thermochemistry is one part of the broader field o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium(IV) Oxide–cerium(III) Oxide Cycle
A ceria based thermochemical cycle is a type of two-step thermochemical cycle that uses as oxygen carrier cerium oxides (CeO_2/Ce_2O_3) for synthetic fuel production such as hydrogen or syngas. These cycles are able to obtain either hydrogen (H_2) from the splitting of water molecules (H_2O), or also syngas, which is a mixture of hydrogen (H_2) and carbon monoxide (CO), by also splitting carbon dioxide (CO_2) molecules alongside water molecules. These types of thermochemical cycles are mainly studied for concentrated solar applications. Types of cycles These cycles are based on the two step redox thermochemical cycle. In the first step, a metal oxide, such as ceria, is reduced by providing heat to the material, liberating oxygen. In the second step, a stream of steam oxidises the previously obtained molecule back to its starting state, therefore closing the cycle. Depending on the stoichiometry of the reactions, which is the relation of the reactants and products of the chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particulate Matter
Particulate matter (PM) or particulates are microscopic particles of solid or liquid matter suspended in the air. An ''aerosol'' is a mixture of particulates and air, as opposed to the particulate matter alone, though it is sometimes defined as a subset of aerosol terminology. Sources of particulate matter can be natural or anthropogenic. Particulates have impacts on climate and precipitation that adversely affect human health. Types of atmospheric particles include suspended particulate matter; thoracic and respirable particles; inhalable coarse particles, designated PM, which are coarse particles with a diameter of 10 micrometers (μm) or less; fine particles, designated PM, with a diameter of 2.5 μm or less; ultrafine particles, with a diameter of 100 nm or less; and soot. Airborne particulate matter is a Group 1 carcinogen. Particulates are the most harmful form of air pollution as they can penetrate deep into the lungs and brain from blood streams, ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Efficiency
Fuel efficiency (or fuel economy) is a form of thermal efficiency, meaning the ratio of effort to result of a process that converts chemical energy, chemical potential energy contained in a carrier (fuel) into kinetic energy or Mechanical work, work. Overall fuel efficiency may vary per device, which in turn may vary per application, and this spectrum of variance is often illustrated as a continuous energy profile. Non-transportation applications, such as Industrial sector, industry, benefit from increased fuel efficiency, especially fossil fuel power plants or industries dealing with combustion, such as ammonia production during the Haber process. In the context of transport, fuel economy is the energy efficiency in transportation, energy efficiency of a particular vehicle, given as a ratio of distance traveled per unit of Motor fuel, fuel consumed. It is dependent on several factors including engine efficiency, transmission (mechanics), transmission design, and tire design. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |