|
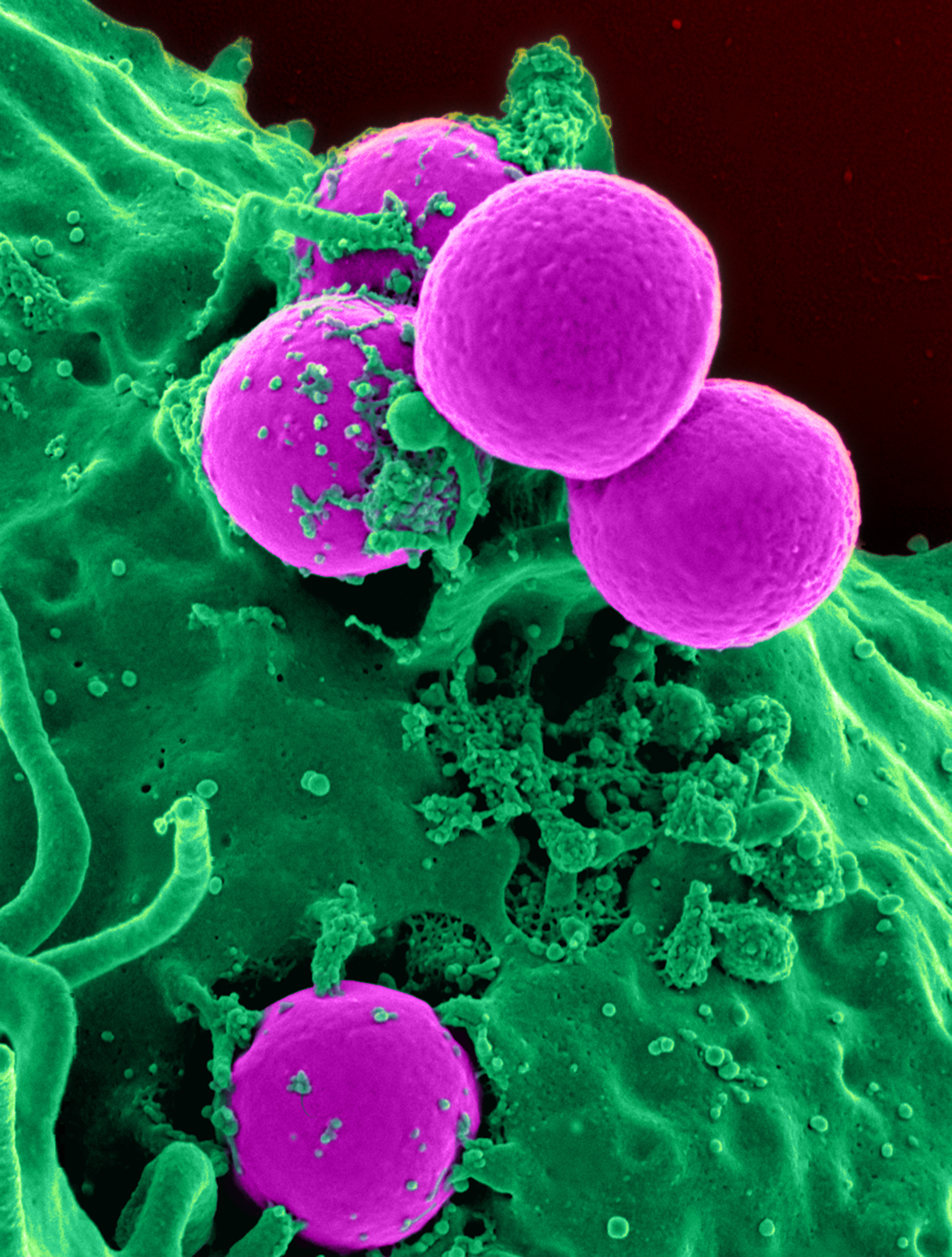
Cefoperazone
Cefoperazone is a third-generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid. It is one of few cephalosporin antibiotics effective in treating ''Pseudomonas'' bacterial infections which are otherwise resistant to these antibiotics. It was patented in 1974 and approved for medical use in 1981. Cefoperazone/sulbactam (Sulperazon) is a co-formulation with sulbactam. Spectrum of bacterial susceptibility Cefoperazone has a broad spectrum of activity and has been used to target bacteria responsible for causing infections of the respiratory and urinary tract, skin, and the female genital tract. The following represents MIC susceptibility data for a few medically significant microorganisms. * ''Haemophilus influenzae'': 0.12 - 0.25 μg/ml * ''Staphylococcus aureus'': 0.125 - 32 μg/ml * ''Streptococcus pneumoniae'': ≤0.007 - 1 μg/ml Adverse effects Cefoperazone contains an ''N''-methylthiotetrazole (NMTT or 1-MTT) side chain. As the antibioti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cefoperazone/sulbactam
Cefoperazone/sulbactam is a combination drug used as an antibiotic. It is effective for the treatment of urinary tract infections. It contains cefoperazone, a β-lactam antibiotic, and sulbactam, a β-lactamase inhibitor Beta-lactamases are a family of enzymes involved in bacterial antimicrobial resistance, resistance to β-lactam antibiotic, beta-lactam antibiotics. In bacterial resistance to beta-lactam antibiotics, the bacteria have beta-lactamase which degra ..., which helps prevent bacteria from breaking down cefoperazone. References Drugs developed by Pfizer Combination antibiotics Antibiotics {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde Dehydrogenase Inhibitors
A disulfiram-like drug is a drug that causes an adverse reaction to alcohol (drug), alcohol leading to nausea, vomiting, alcohol flush reaction, flushing, dizziness, throbbing headache, chest pain, chest and abdominal pain, abdominal discomfort, and general hangover-like symptoms among others. These effects are caused by accumulation of acetaldehyde, a major but toxic metabolite of alcohol formed by the enzyme alcohol dehydrogenase. The reaction has been variously termed a ''disulfiram-like reaction'', ''alcohol intolerance'', and ''acetaldehyde syndrome''.Mutalik, M., & Sanghavi, D. (2014). ''Review of Drug Interactions: A Comprehensive Update''. The prototypical drug of this group is disulfiram (brand name Antabuse), which acts as an acetaldehyde dehydrogenase enzyme inhibitor, inhibitor, preventing the metabolism of acetaldehyde into acetic acid, and is used in the treatment of alcoholism. A variety of other drugs cause disulfiram-like reactions upon consumption of alcohol a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephalosporin
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus '' Acremonium'', which was previously known as ''Cephalosporium''. Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964. Discovery The aerobic mold which yielded cephalosporin C was found in the sea near a sewage outfall in Su Siccu, by Cagliari harbour in Sardinia, by the Italian pharmacologist Giuseppe Brotzu in July 1945. Structure Cephalosporin contains a 6-membered dihydrothiazine ring. Substitutions at position 3 generally affect pharmacology; substitutions at position 7 affect antibacterial activity, but these cases are not always true. Medical uses Cephalosporins can be indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. First-generation cephalosporins are active predominant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulbactam
Sulbactam is a β-lactamase inhibitor. This drug is given in combination with β-lactam antibiotics to inhibit β-lactamase, an enzyme produced by bacteria that destroys the antibiotic An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...s. It was patented in 1977 and approved for medical use in 1986. Medical uses The combination ampicillin/sulbactam (Unasyn) is available in the United States. The combination cefoperazone/sulbactam (Sulperazon) is available in many countries but not in the United States. The co-packaged combination sulbactam/durlobactam was approved for medical use in the United States in May 2023. Mechanism Sulbactam is primarily used as a suicide inhibitor of β-lactamase, shielding more potent beta-lactams such as ampicillin. Sulbactam itself contains a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephalosporin Antibiotics
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus '' Acremonium'', which was previously known as ''Cephalosporium''. Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964. Discovery The aerobic mold which yielded cephalosporin C was found in the sea near a sewage outfall in Su Siccu, by Cagliari harbour in Sardinia, by the Italian pharmacologist Giuseppe Brotzu in July 1945. Structure Cephalosporin contains a 6-membered dihydrothiazine ring. Substitutions at position 3 generally affect pharmacology; substitutions at position 7 affect antibacterial activity, but these cases are not always true. Medical uses Cephalosporins can be indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. First-generation cephalosporins are active predominantly ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfiram
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol (drinking alcohol). Disulfiram works by Enzyme inhibition, inhibiting the enzyme aldehyde dehydrogenase (specifically ALDH2), causing many of the effects of a hangover to be felt immediately following alcohol (drug), alcohol consumption. Disulfiram plus alcohol, even small amounts, produces flushing, throbbing in the head and neck, a throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst, chest pain, palpitation, dyspnea, shortness of breath, hyperventilation, tachycardia, fast heart rate, hypotension, low blood pressure, Syncope (medicine), fainting, marked uneasiness, weakness, vertigo, blurred vision, and confusion. In severe reactions there may be respiratory depression, cardiovascular collapse, arrhythmia, abnormal heart rhythms, myocardial infarction, heart attack, acute congestive heart failure, unconsciousness, convulsi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde Dehydrogenase
Aldehyde dehydrogenases () are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes (R–C(=O)) to carboxylic acids (R–C(=O)). The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes. Function Aldehyde dehydrogenase is a polymorphic enzyme responsible for the oxidation of aldehydes to carboxylic acids. There are three different classes of these enzymes in mammals: class 1 (low ''K''m, cytosolic), class 2 (low ''K''m, mitochondrial), and class 3 (high ''K''m, such as those expressed in tumors, stomach, and cornea). In all three classes, constitutive and inducible forms exist. ALDH1 and ALDH2 are the most important enzymes for aldehyde oxidation, and both are tetrameric enzymes composed of 54 kDa subunits. These enzymes are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetamides
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is an amide derived from ammonia and acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound ''N'',''N''-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide. Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations. Acetamide is also a naturally occurring mineral with the IMA symbol: Ace. Production Laboratory scale Acetamide can be produced in the laboratory from ammonium acetate by dehydration: : H4CH3CO2] → CH3C(O)NH2 + H2O Alternatively acetamide can be obtained in excellent yield via ammonolysis of acetylacetone under conditions commonly used in reductive amination. It can also be made from anhydrous acetic acid, acetonitrile and very well dried hydrogen chloride gas, u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketopiperazines
A diketopiperazine (DKP), also known as a ''dioxopiperazine'' or ''piperazinedione'', is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, they are not ketones, but amides. Three regioisomers are possible, differing in the locations of the carbonyl groups. * One isomer is an oxamide obtained from ethylenediamine. * 2,5-Diketopiperazines are cyclodipeptides often obtainable via condensation of two α-amino acids. * 2,6-Diketopiperazines may be viewed as cyclized imide derivatives derived from iminodiacetic acids. Of these three isomeric diketopiperazines, the 2,5-derivatives have attracted the greatest interest. Due to their appearance in biologically active natural products, medicinal chemists have been inspired to use DKPs to circumvent the poor physical and metabolic properties of peptides in the course of drug discovery. Natural sources DKPs are synthesized by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazoles
A tetrazole is a synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound - a whitish crystalline powder with the formula CH2N4, of which three isomers exist. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Phosphorus analogs do not have the same electronic nature, with 1''H''-tetraphosphole having a more pyramidal geometry of the phosphorus at position 1. Instead, it is the anionic tetraphospholides that are aromatic. Strongly inductively electron-withdrawing functional groups attached ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |