|
Caspase-5
Caspase 5 is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue, and belongs to a family of cysteine proteases called caspases. It is an inflammatory caspase, along with caspase 1, caspase 4 and the murine caspase 4 homolog caspase 11, and has a role in the immune system. See also * The Proteolysis Map * Caspase References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ... online database for peptidases and their inhibitorsC14.008 EC 3.4.22 Caspases {{gene-11-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
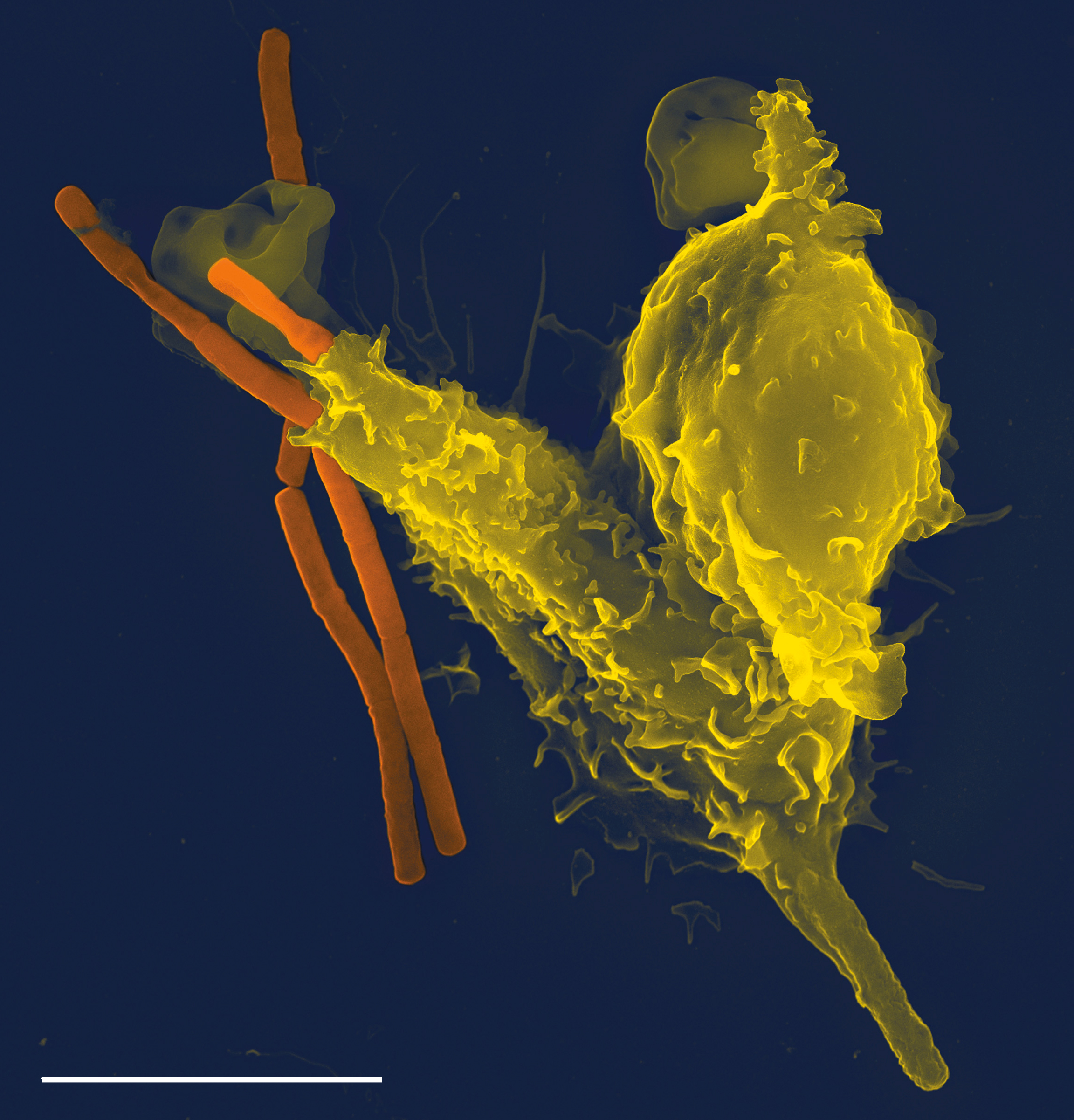
Caspase 11
Murine caspase-11, and its human homologs caspase-4 and caspase-5, are mammalian intracellular receptor proteases activated by TLR4 and TLR3 signaling during the innate immune response. Caspase-11, also termed the non-canonical inflammasome, is activated by TLR3/TLR4- TRIF signaling and directly binds cytosolic lipopolysaccharide (LPS), a major structural element of Gram-negative bacterial cell walls. Activation of caspase-11 by LPS is known to cause the activation of other caspase proteins, leading to septic shock, pyroptosis, and often organismal death. History LPS is a known activator of innate immune responses. Extracellular LPS binds specifically to the cell surface receptor TLR4. LPS binding to TLR4 subsequently causes initiation of the MyD88 and TRIF signaling pathways, leading to expression of pro- inflammatory molecules and cytokines. These inflammatory mediators cause host toxic shock and sepsis as a result of an overactive immune response to LPS. Until recen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. .html" ;"title="/sup>">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine Protease
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad. Discovered by Gopal Chunder Roy in 1873, the first cysteine protease to be isolated and characterized was papain, obtained from ''Carica papaya''. Cysteine proteases are commonly encountered in fruits including the papaya, pineapple, fig and kiwifruit. The proportion of protease tends to be higher when the fruit is unripe. In fact, the latex of dozens of different plant families are known to contain cysteine proteases. Cysteine proteases are used as an ingredient in meat tenderizers. Classification The MEROPS protease classification system counts 14 superfamilies plus several currently unassigned families (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evolutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 1
Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells. Cellular expression Caspase-1 is evolutionarily conserved in many eukaryotes of the Kingdom Animalia. Due to its role in the inflammatory immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 4
Caspase 4 is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue (LEVD-), and belongs to a family of cysteine proteases called caspases. The function of caspase 4 is not fully known, but it is believed to be an inflammatory caspase, along with caspase 1, caspase 5 (and the murine homolog caspase 11), with a role in the immune system The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint .... The Nonsteroidal anti-inflammatory drug, anti-inflammatory drug indoprofen is an inhibitor of the activity of the caspase-4 enzyme. See also * The Proteolysis Map * Caspase References External links * The MEROPS online database for peptidases and their inhibitorsC14.007 EC 3.4.22 Caspases {{gene-11-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Murine
The Old World rats and mice, part of the subfamily Murinae in the family Muridae, comprise at least 519 species. Members of this subfamily are called murines. In terms of species richness, this subfamily is larger than all mammal families except the Cricetidae and Muridae, and is larger than all mammal orders except the bats and the remainder of the rodents. Description The Murinae are native to Africa, Europe, Asia, and Australia. They are terrestrial placental mammals. They have also been introduced to all continents except Antarctica, and are serious pest animals. This is particularly true in island communities where they have contributed to the endangerment and extinction of many native animals. Two prominent murine species have become vital laboratory animals: the brown rat and house mouse are both used as medical subjects. The murines have a distinctive molar pattern that involves three rows of cusps instead of two, the primitive pattern seen most frequently in mur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology (biology)
In biology, homology is similarity due to shared ancestry between a pair of structures or genes in different taxa. A common example of homologous structures is the forelimbs of vertebrates, where the wings of bats and birds, the arms of primates, the front flippers of whales and the forelegs of four-legged vertebrates like dogs and crocodiles are all derived from the same ancestral tetrapod structure. Evolutionary biology explains homologous structures adapted to different purposes as the result of descent with modification from a common ancestor. The term was first applied to biology in a non-evolutionary context by the anatomist Richard Owen in 1843. Homology was later explained by Charles Darwin's theory of evolution in 1859, but had been observed before this, from Aristotle onwards, and it was explicitly analysed by Pierre Belon in 1555. In developmental biology, organs that developed in the embryo in the same manner and from similar origins, such as from matching p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune System
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against virus infections. Other basic immune mechanisms evolved in ancient plants and animals and remain in their modern descendants. These mechanisms include phagocytosis, antimicrobial pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Proteolysis Map
The Proteolysis MAP (PMAP) is an integrated web resource focused on proteases. Rationale PMAP is to aid the protease researchers in reasoning about proteolytic networks and metabolic pathways. History and funding PMAP was originally created at the Burnham Institute for Medical Research, La Jolla, California. In 2004 the National Institutes of Health (NIH) selected a team led by Jeffrey W. Smith, to establish the Center on Proteolytic Pathways (CPP). As part of the NIH Roadmap for Biomedical research, the center develops technology to study the behavior of proteins and to disburse that knowledge to the scientific community at large. Focal point Proteases are a class of enzymes that regulate much of what happens in the human body, both inside the cell and out, by cleaving peptide bonds in proteins. Through this activity, they govern the four essential cell functions: differentiation, motility, division and cell death — and activate important extracellular episodes, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |