|
Cannabis Flower Essential Oil
Cannabis flower essential oil, also known as hemp essential oil, is an essential oil obtained by steam distillation from the flowers, panicles (flower cluster), stem, and upper leaves of the hemp plant (''Cannabis sativa L''.). Hemp essential oil is distinct from hemp seed oil (hemp oil) and hash oil: the former is a vegetable oil that is cold-pressed from the seeds of low-THC varieties of hemp, the latter is a THC-rich extract of dried female hemp flowers (marijuana) or resin ( hashish). A pale yellow liquid, cannabis flower essential oil is a volatile oil that is a mixture of monoterpenes, sesquiterpenes, and other terpenoid compounds. The typical scent of hemp results from about 140 different terpenoids. The essential oil is manufactured from both low-THC ("fibre-type") and high-THC ("drug-type") varieties of hemp. As most of the phytocannabinoids are nearly insoluble in water, hemp essential oil contains only traces of cannabinoids. Even in "drug-type" hemp, the THC content ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabis Sativa - Köhler–s Medizinal-Pflanzen-026
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively, ''C. ruderalis'' may be included within ''C. sativa'', all three may be treated as subspecies of ''C. sativa'', or ''C. sativa'' may be accepted as a single undivided species. The genus is widely accepted as being indigenous to and originating from Asia. The plant is also known as hemp, although this term is often used to refer only to varieties of ''Cannabis'' cultivated for non-drug use. Cannabis has long been used for hemp fibre, hemp seeds and their oils, hemp leaves for use as vegetables and as juice, medicinal purposes, and as a recreational drug. Industrial hemp products are made from cannabis plants selected to produce an abundance of fibre. Various cannabis strains have been bred, often selectively to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpineol
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil. Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. β- and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent. : Terpineol has a pleasant odor similar to lilac and is a common ingredient in perfumes, cosmetics, and flavors. α-Terpineol is one of the two most abundant aroma constituents of lapsang souchong tea; the α-terpineol originates in the pine smoke used to dry the tea. (+)-α-terpineol is a chemical constituent of skullcap. Synthesis and biosynthesis Although it is naturally occurring, terpineol is commonly manufactured from alpha-pinene, which is hydrated in the presence of sulfuric acid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulegone
Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as ''Nepeta cataria'' (catnip), ''Mentha piperita'', and pennyroyal. It is classified as a monoterpene. Pulegone is a clear colorless oily liquid and has a pleasant odor similar to pennyroyal, peppermint and camphor. It is used in flavoring agents, in perfumery, and in aromatherapy. Toxicology It was reported that the chemical is toxic to rats if a large quantity is consumed. Pulegone is also an insecticide − the most powerful of three insecticides naturally occurring in many mint species. As of October 2018, the FDA withdrew authorization for the use of pulegone as a synthetic flavoring substance for use in food, but that naturally-occurring pulegone can continue to be used. Sources * Creeping charlie * ''Mentha longifolia'' * ''Mentha suaveolens'' * Pennyroyal * Peppermint *'' Schizonepeta tenuifolia'' *''Bursera graveolens'' See also * Menthofuran * Ment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eucalyptol
Eucalyptol is a monoterpenoid. A colorless liquid, it is a bicyclic ether. Eucalyptol has a fresh mint-like smell and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up ~70% - 90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, ''o''-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification. In 1870, F. S. Cloez identified and ascribed the name "eucalyptol" to the dominant portion of ''Eucalyptus globulus'' oil. Uses Because of its pleasant, spicy aroma and taste, eucalyptol is used in flavorings, fragrances, and cosmetics. Cineole-based eucalyptus oil is used as a flavouring at low levels (0.002%) in various products, including baked goods, confectionery, meat products, and beverages. In a 1994 report released by five top cigarette companies, eucalyptol was listed as one of the 599 additives to cigarettes. It is claimed to be added to improve the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cineole
Eucalyptol is a monoterpenoid. A colorless liquid, it is a bicyclic ether. Eucalyptol has a fresh mint-like smell and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up ~70% - 90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, ''o''-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification. In 1870, F. S. Cloez identified and ascribed the name "eucalyptol" to the dominant portion of ''Eucalyptus globulus'' oil. Uses Because of its pleasant, spicy aroma and taste, eucalyptol is used in flavorings, fragrances, and cosmetics. Cineole-based eucalyptus oil is used as a flavouring at low levels (0.002%) in various products, including baked goods, confectionery, meat products, and beverages. In a 1994 report released by five top cigarette companies, eucalyptol was listed as one of the 599 additives to cigarettes. It is claimed to be added to improve the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpinene
The terpinenes are a group of isomeric hydrocarbons that are classified as monoterpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source but has been prepared from sabinene. γ-Terpinene and δ-terpinene (also known as terpinolene) have been isolated from a variety of plant sources. They are all colorless liquids with a turpentine-like odor. Production and uses α-Terpinene is produced industrially by acid-catalyzed rearrangement of α-pinene. It has perfume and flavoring properties but is mainly used to confer pleasant odor to industrial fluids. Hydrogenation gives the saturated derivative ''p''-menthane. Biosynthesis of α-terpinene The biosynthesis of α-terpinene and other terpenoids starts with the isomerization of geranyl pyrophosphate to linalyl pyroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
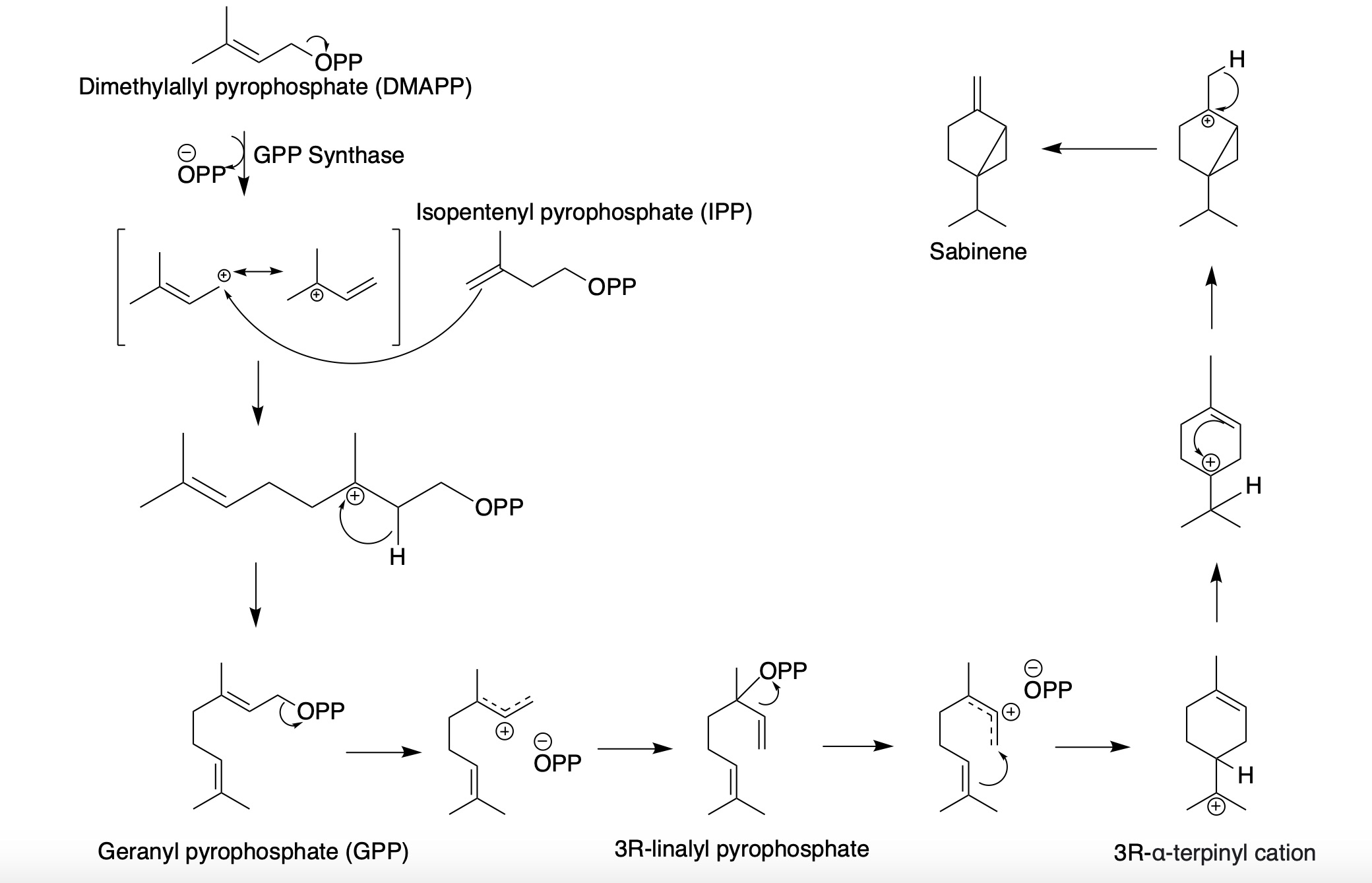
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, ''Laurus nobilis'', and ''Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomers. It is biosynthesized from the common terpenoid precursor, geranyl pyrophosphate (GPP) that undergoes polycyclization catalyzed by sabinene synthase (SabS). GPP is formed from the terpenoid synthesis pathway with the starter units, isopentenyl pyrophosphate (IPP) and dimethylallyl py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humulene
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of ''Humulus lupulus'' (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants. Occurrence Humulene is one of the components of the essential oil from the flowering cone of the hops plant, ''Humulus lupulus'', from which it derives its name. The concentration of humulene varies among different varieties of the plant but can be up to 40% of the essential oil. Humulene and its reaction products in the brewing process of beer gives many beers their “hoppy” aroma. Noble hop varieties have been found to have higher levels of humulene, while oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpinolene
The terpinenes are a group of isomeric hydrocarbons that are classified as monoterpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source but has been prepared from sabinene. γ-Terpinene and δ-terpinene (also known as terpinolene) have been isolated from a variety of plant sources. They are all colorless liquids with a turpentine-like odor. Production and uses α-Terpinene is produced industrially by acid-catalyzed rearrangement of α-pinene. It has perfume and flavoring properties but is mainly used to confer pleasant odor to industrial fluids. Hydrogenation gives the saturated derivative ''p''-menthane. Biosynthesis of α-terpinene The biosynthesis of α-terpinene and other terpenoids starts with the isomerization of geranyl pyrophosphate to linalyl pyroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ocimene
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is ''cis-''3,7-dimethyl-1,3,7-octatriene. β-Ocimene is ''trans-''3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, ''cis'' and ''trans,'' with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinene
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers. Pinenes are also found in many non-coniferous plants such as camphorweed (''Heterotheca'') and big sagebrush (''Artemisia tridentata''). Isomers Biosynthesis α-Pinene and β-pinene are both produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate followed by loss of a proton from the carbocation equivalent. Researchers at the Georgia Institute of Technology and the Joint BioEnergy Institute have been able to synthetically produce pinene with a bacterium. Plants Alpha-pinene is the most widely encountered terpenoid in nature and is highly repellent to insects. Alpha-pinene appears in conifers and numerous other plants. Pinene is a major component of the essential oil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |