|
Caloric Fluid
The caloric theory is an obsolete scientific theory that heat consists of a self-repellent fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also thought of as a weightless gas that could pass in and out of pores in solids and liquids. The "caloric theory" was superseded by the mid-19th century in favor of the mechanical theory of heat, but nevertheless persisted in some scientific literature—particularly in more popular treatments—until the end of the 19th century. Early history In the history of thermodynamics, the initial explanations of heat were thoroughly confused with explanations of combustion. After J. J. Becher and Georg Ernst Stahl introduced the phlogiston theory of combustion in the 17th century, phlogiston was thought to be the ''substance of heat.'' There is one version of the caloric theory that was introduced by Antoine Lavoisier. Prior to Lavoisier's caloric theory, published references concerning heat and its existence, outsi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Obsolete Scientific Theory
This list catalogs well-accepted theories in science and pre-scientific natural philosophy and natural history which have since been superseded by scientific theories. Many discarded explanations were once supported by a scientific consensus, but replaced after more empirical information became available that identified flaws and prompted new theories which better explain the available data. Pre-modern explanations originated before the scientific method, with varying degrees of empirical support. Some theories are discarded in their entirety, such as the replacement of the phlogiston theory by energy and thermodynamics. Some theories known to be incomplete or in some ways incorrect are still used. For example, Newtonian classical mechanics is accurate enough for practical calculations at everyday distances and velocities, and it is still taught in schools. The more complicated relativistic mechanics must be used for long distances and velocities nearing the speed of light, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pierre Prévost (physicist)
Pierre Prévost (; 3 March 1751 – 8 April 1839) was a Genevan philosopher and physicist. In 1791 he explained Pictet's experiment by arguing that all bodies radiate heat, no matter how hot or cold they are. Life Son of a Protestant clergyman in Geneva in the Republic of Geneva, he was born in that city and was educated for a clerical career. However, he abandoned it for law, and this too he quickly deserted to devote himself to education and to travelling. He became close friends with Jean Jacques Rousseau and, a little later, with Dugald Stewart, having previously distinguished himself as a translator of and commentator on Euripides. Frederick II of Prussia secured him in 1780 as professor of philosophy and made him member of the Akademie der Wissenschaften in Berlin. He there became acquainted with Joseph Louis Lagrange and was thus led to turn his attention to physical science. After some years spent on political economy and on the principles of the fine arts (in connect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isaac Newton
Sir Isaac Newton (25 December 1642 – 20 March 1726/27) was an English mathematician, physicist, astronomer, alchemist, theologian, and author (described in his time as a "natural philosopher"), widely recognised as one of the greatest mathematicians and physicists and among the most influential scientists of all time. He was a key figure in the philosophical revolution known as the Enlightenment. His book (''Mathematical Principles of Natural Philosophy''), first published in 1687, established classical mechanics. Newton also made seminal contributions to optics, and shares credit with German mathematician Gottfried Wilhelm Leibniz for developing infinitesimal calculus. In the , Newton formulated the laws of motion and universal gravitation that formed the dominant scientific viewpoint for centuries until it was superseded by the theory of relativity. Newton used his mathematical description of gravity to derive Kepler's laws of planetary motion, account for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pierre-Simon Laplace
Pierre-Simon, marquis de Laplace (; ; 23 March 1749 – 5 March 1827) was a French scholar and polymath whose work was important to the development of engineering, mathematics, statistics, physics, astronomy, and philosophy. He summarized and extended the work of his predecessors in his five-volume ''Mécanique céleste'' (''Celestial Mechanics'') (1799–1825). This work translated the geometric study of classical mechanics to one based on calculus, opening up a broader range of problems. In statistics, the Bayesian interpretation of probability was developed mainly by Laplace. Laplace formulated Laplace's equation, and pioneered the Laplace transform which appears in many branches of mathematical physics, a field that he took a leading role in forming. The Laplacian differential operator, widely used in mathematics, is also named after him. He restated and developed the nebular hypothesis of the origin of the Solar System and was one of the first scientists to sugges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
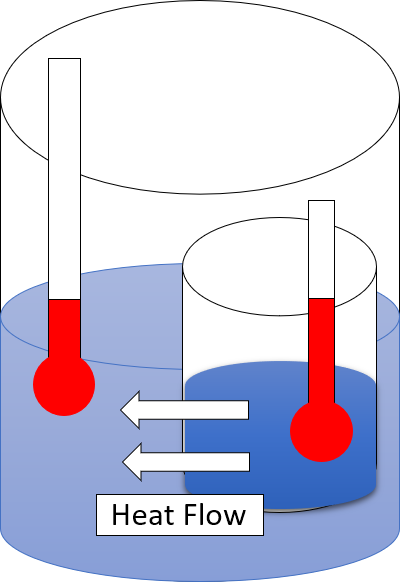
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and Energy transformation, energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag. In general, an engine is any machine that converts energy to mechanical work. Heat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is reversible, and there is no generation of entropy. (In other words, entropy is conserved; entropy is only transferred between the thermal reservoirs and the system without gain or loss of it.) When work is applied to the system, heat moves from the cold to hot reser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Law
The gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between pressure, volume and temperature of a sample of gas could be obtained which would hold to approximation for all gases. Boyle's law In 1662 Robert Boyle studied the relationship between volume and pressure of a gas of fixed amount at constant temperature. He observed that volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature. Boyle's law, published in 1662, states that, at constant temperature, the product of the pressure and volume of a given mass of an ideal gas in a closed system is always constant. It can be verified experimentally using a pressure gauge and a variable volume container. It can also be derived from the kinetic theory of gases: if a container, with a fixed number of molecules inside, is reduced in volume, more molecules will strike a given area of the sides of the container per unit time, causing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition At the phase transition point for a substance, for instance the boiling point, the two phases involved - liquid and vapor, have identic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of particles in matter. Thermal radiation is generated when heat from the movement of charges in the material (electrons and protons in common forms of matter) is converted to electromagnetic radiation. All matter with a temperature greater than absolute zero emits thermal radiation. At room temperature, most of the emission is in the infrared (IR) spectrum. Particle motion results in charge-acceleration or dipole oscillation which produces electromagnetic radiation. Infrared radiation emitted by animals (detectable with an infrared camera) and cosmic microwave background radiation are examples of thermal radiation. If a radiation object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends solely on the object's temperature. Wien's displacement law de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). The definition of length (cubed) is interrelated with volume. The volume of a container is generally understood to be the capacity of the container; i.e., the amount of fluid (gas or liquid) that the container could hold, rather than the amount of space the container itself displaces. In ancient times, volume is measured using similar-shaped natural containers and later on, standardized containers. Some simple three-dimensional shapes can have its volume easily calculated using arithmetic formulas. Volumes of more complicated shapes can be calculated with integral calculus if a formula exists for the shape's boundary. Zero-, one- and two-dimensional objects have no volume; in fourth and higher dimensions, an analogous concept to the normal vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Theory
Atomic theory is the scientific theory that matter is composed of particles called atoms. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. According to this idea, if one were to take a lump of matter and cut it into ever smaller pieces, one would eventually reach a point where the pieces could not be further cut into anything smaller. Ancient Greek philosophers called these hypothetical ultimate particles of matter ''atomos'', a word which meant "uncut". In the early 1800s, the scientist John Dalton noticed that chemical substances seemed to combine and break down into other substances by weight in proportions that suggested that each chemical element is ultimately made up of tiny indivisible particles of consistent weight. Shortly after 1850, certain physicists developed the kinetic theory of gases and of heat, which mathematically modelled the behavior of gases by assuming that they were made of particles. In the early 20th century, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)