|
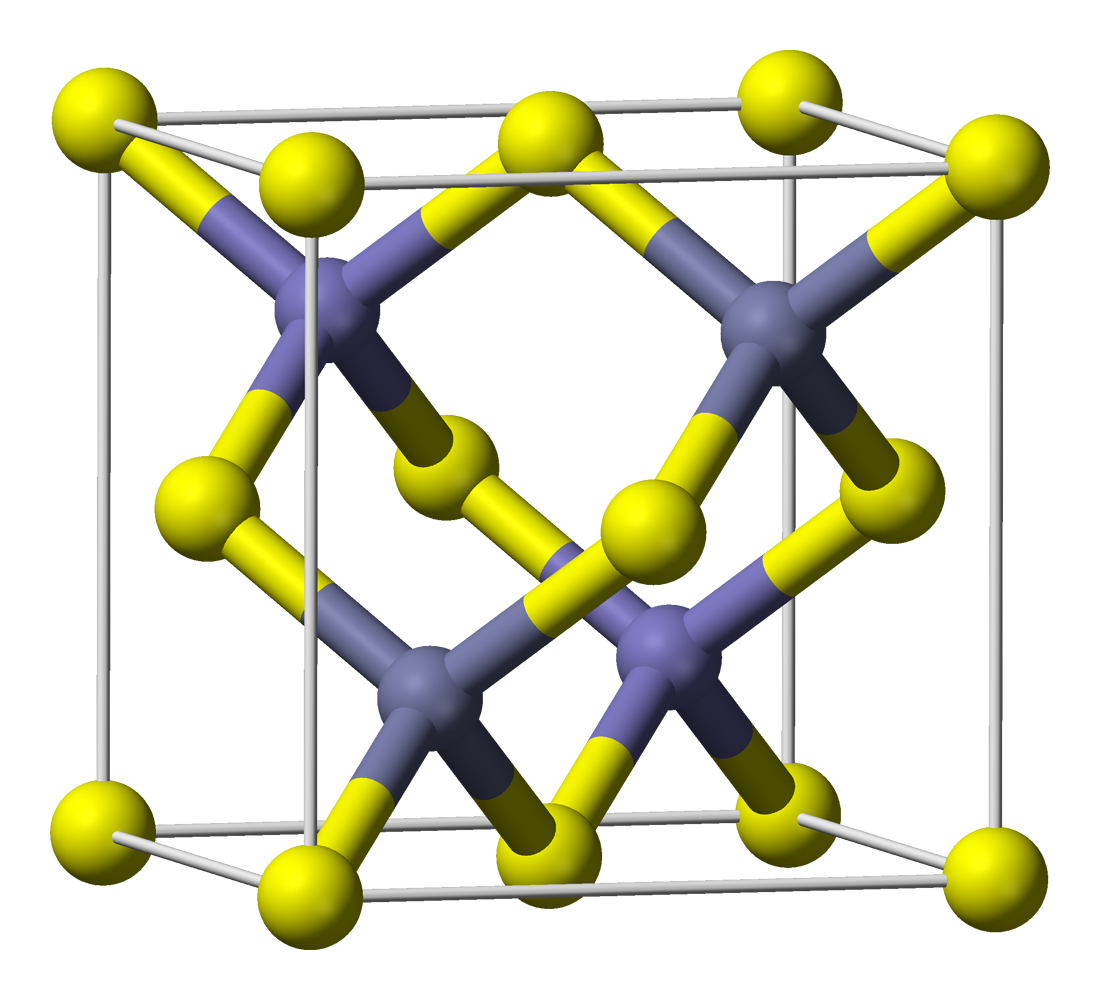
Calcium Tungstate
Scheelite is a calcium tungstate mineral with the chemical formula calcium, Catungsten, Woxygen, O4. It is an important ore of tungsten (wolfram). Scheelite is originally named after Swedish chemist K. Scheele (1742-1786). Well-formed crystals are sought by collectors and are occasionally fashioned into gemstones when suitably free of flaws. Scheelite has been Chemical synthesis, synthesized using the Czochralski process; the material produced may be used to diamond simulant, imitate diamond, as a scintillator, or as a solid, solid-state lasing medium. It was also used in Radium#Luminescent paint, radium paint in the same fashion as was zinc sulphide, and Thomas Edison invented a fluoroscope with a calcium tungstate-coated screen, making the images six times brighter than those with barium platinocyanide; the latter chemical allowed Wilhelm Röntgen, Röntgen to discover X-rays in early November 1895. Properties Its crystals are in the tetragonal crystal system, appearing as dip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Sulphide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mohs Scale Of Hardness
The Mohs scale of mineral hardness () is a Qualitative property, qualitative ordinal scale, from 1 to 10, characterizing scratch hardness, scratch resistance of various minerals through the ability of harder material to scratch softer material. The scale was introduced in 1812 by the German geologist and Mineralogy, mineralogist Friedrich Mohs, in his book ''"Versuch einer Elementar-Methode zur naturhistorischen Bestimmung und Erkennung der Fossilien"''; it is one of several definitions of Hardness comparison, hardness in materials science, some of which are more quantitative. The method of comparing hardness by observing which minerals can scratch others is of great antiquity, having been mentioned by Theophrastus in his treatise ''On Stones'', , followed by Pliny the Elder in his ''Natural History (Pliny), Naturalis Historia'', . The Mohs scale is useful for identification of minerals in the field, but is not an accurate predictor of how well materials endure in an industrial s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conchoidal Fracture
Conchoidal fracture describes the way that brittle materials break or fracture when they do not follow any natural planes of separation. Mindat.org defines conchoidal fracture as follows: "a fracture with smooth, curved surfaces, typically slightly concave, showing concentric undulations resembling the lines of growth of a shell".Conchoidal fracture at Materials that break in this way include quartz, chert, flint, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage."Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica." Diamond and graphite provide examples of cleavage. Both are composed solely of a single element, carbon. But in diamond, each carbon atom is bonded to four others in a tetrahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lustre (mineralogy)
Lustre (British English) or luster (American English; see spelling differences) is the way light interacts with the surface of a crystal, rock, or mineral. The word traces its origins back to the Latin ''lux'', meaning "light", and generally implies radiance, gloss, or brilliance. A range of terms are used to describe lustre, such as ''earthy'', ''metallic'', ''greasy'', and ''silky''. Similarly, the term ''vitreous'' (derived from the Latin for glass, ''vitrum'') refers to a glassy lustre. A list of these terms is given below. Lustre varies over a wide continuum, and so there are no rigid boundaries between the different types of lustre. (For this reason, different sources can often describe the same mineral differently. This ambiguity is further complicated by lustre's ability to vary widely within a particular mineral species). The terms are frequently combined to describe intermediate types of lustre (for example, a "vitreous greasy" lustre). Some minerals exhibit unu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal System
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family. The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (albeit there are many exceptions). Classifications Crystals can be classified in three ways: lattice systems, crystal systems and crystal families. The various classifications are often confused: in particular the trigonal crystal system is often confused with the rhombohedral lattice system, and the term "crystal system" is sometimes used to mean "lattic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-rays
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-ray wavelengths are shorter than those of ultraviolet rays and longer than those of gamma rays. There is no universally accepted, strict definition of the bounds of the X-ray band. Roughly, X-rays have a wavelength ranging from 10 nanometers to 10 picometers, corresponding to frequencies in the range of 30 petahertz to 30 exahertz ( to ) and photon energies in the range of 100 eV to 100 keV, respectively. X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelm Röntgen
Wilhelm Conrad Röntgen (; ; 27 March 184510 February 1923) was a German mechanical engineer and physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range known as X-rays or Röntgen rays, an achievement that earned him the inaugural Nobel Prize in Physics in 1901.Novelize, Robert. ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. 1997. p. 1. In honour of Röntgen's accomplishments, in 2004 the International Union of Pure and Applied Chemistry (IUPAC) named element 111, roentgenium, a radioactive element with multiple unstable isotopes, after him. The unit of measurement roentgen was also named after him. Biographical history Education He was born to Friedrich Conrad Röntgen, a German merchant and cloth manufacturer, and Charlotte Constanze Frowein. At age three his family moved to the Netherlands where his family lived. Röntgen attended high school at Utrecht Technical School in Utrecht, Netherla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinocyanide
Platinocyanide, also known as tetracyanoplatinate (IUPAC), cyanoplatinate, or platinocyanate, is a polyatomic ion with the molecular formula t(CN)4sup>2−. The name also applies to compounds containing this ion, which are salts of the hypothetical platinocyanic acid (sometimes platinocyanhydric acid). Barium platinocyanide, Ba t(CN)4is a phosphor and a scintillator. It fluoresces in the presence of x-rays and gamma rays. It was important in the discovery of X-rays, and in the development of the fluoroscope. One platinocyanide salt, Krogmann's salt (dipotassium tetracyanoplatinate bromide trihydrate), has unusually high electric conductance. Cyano complexes Phosphors and scintillators Cyanides Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ... Cyanometallates {{inorganic- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |