|
Calcitonin Gene-related Peptide Antagonist
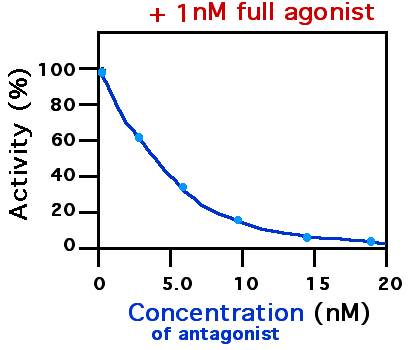
Calcitonin gene-related peptide (CGRP) receptor antagonists are a class of drugs that act as antagonists of the calcitonin gene-related peptide receptor (CGRPR). Several monoclonal antibodies which binds to the CGRP receptor or peptide have been approved for prevention of migraine. Three small molecule CGRPR antagonists are approved in the U.S. as antimigraine agents. Drugs of this class have also been investigated for use in osteoarthritis. Examples Non-peptide small molecules * Ubrogepant is approved for acute treatment of migraines *Rimegepant (BMS-927711) is approved for acute and preventative treatment of migraines * Atogepant (AGN-241689) is approved for preventative treatment of migraines *Telcagepant (MK-0974), reached phase III clinical trials; development discontinued in 2011. *Olcegepant (BIBN-4096BS) is a drug candidate * BI 44370 TA (BI 44370) * MK-3207 * SB-268262 Monoclonal antibodies targeting the CGRP receptor * Erenumab (AMG-334) is approved for prevention of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Eptinezumab
Eptinezumab, sold under the brand name Vyepti, is a medication used for the preventive treatment of migraine in adults. It is a monoclonal antibody that targets calcitonin gene-related peptides (CGRP) alpha and beta. It is administered by intravenous infusion. Eptinezumab was approved for medical use in the United States in February 2020. History The U.S. Food and Drug Administration (FDA) approved eptinezumab based primarily on evidence from two clinical trials (Trial 1/ NCT02559895 and Trial 2/ NCT02974153) of 1741 subjects with chronic or episodic migraine headaches. Trials were conducted at 212 sites in United States, Georgia, Russia, Ukraine and European Union. The benefit and side effects of eptinezumab were evaluated in two clinical trials of adult subjects 18 – 71 years of age with a history of migraine headaches. The trials had similar designs. Trial 1 enrolled subjects with a history of episodic migraine headaches and Trial 2 enrolled subjects with chronic migra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimigraine Drugs
Antimigraine drugs are medications intended to reduce the effects or intensity of migraine headache. They include drugs for the treatment of acute migraine symptoms as well as drugs for the prevention of migraine attacks. Treatment of acute symptoms Examples of specific antimigraine drug classes include triptans (first line option), ergot alkaloids, ditans and gepants. Migraines can also be treated with unspecific analgesics such as nonsteroidal anti-inflammatory drugs Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of ... (NSAIDs) or acetaminophen. Opioids are not recommended for treatment of migraines. Triptans The triptan drug class includes 1st generation sumatriptan (which has poor bioavailability), and second generation zolmitriptan. Due to their safety, efficacy and selectiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (journal)
''Cell'' is a peer-reviewed scientific journal publishing research papers across a broad range of disciplines within the life sciences. Areas covered include molecular biology, cell biology, systems biology, stem cells, developmental biology, genetics and genomics, proteomics, cancer research, immunology, neuroscience, structural biology, microbiology, virology, physiology, biophysics, and computational biology. The journal was established in 1974 by Benjamin LewinElsevier: ''Cell'': Home (accessed 12 December 2008) and is published twice monthly by , an imprint of |
Eptinezumab
Eptinezumab, sold under the brand name Vyepti, is a medication used for the preventive treatment of migraine in adults. It is a monoclonal antibody that targets calcitonin gene-related peptides (CGRP) alpha and beta. It is administered by intravenous infusion. Eptinezumab was approved for medical use in the United States in February 2020. History The U.S. Food and Drug Administration (FDA) approved eptinezumab based primarily on evidence from two clinical trials (Trial 1/ NCT02559895 and Trial 2/ NCT02974153) of 1741 subjects with chronic or episodic migraine headaches. Trials were conducted at 212 sites in United States, Georgia, Russia, Ukraine and European Union. The benefit and side effects of eptinezumab were evaluated in two clinical trials of adult subjects 18 – 71 years of age with a history of migraine headaches. The trials had similar designs. Trial 1 enrolled subjects with a history of episodic migraine headaches and Trial 2 enrolled subjects with chronic migra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galcanezumab
Galcanezumab, sold under the brand name Emgality, is a humanized monoclonal antibody used for the prevention of migraine. It is also used for cluster headaches. Common side effects include pain or redness at the site of injection. Other side effects may include hypersensitivity reactions. A substance called calcitonin gene-related peptide (CGRP) has been shown to be involved in the development of migraine by widening blood vessels in the brain. Galcanezumab is a monoclonal antibody (a type of protein) designed to attach to and block CGRP, thereby helping blood vessels to return to their normal size. This will stop the symptoms of migraine. This drug was developed by Eli Lilly. It was approved for medical use in the United States and in the European Union in 2018, becoming the third calcitonin gene-related peptide (CGRP) inhibitor to do so. When used for migraines it costs about 7,000 per year in the United States . History In September 2018, galcanezumab-gnlm was approved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fremanezumab
Fremanezumab, sold under the brand name Ajovy, is a medication used to prevent migraines in adults. It is given by injection under the skin. The most common side effect is pain and redness at the site of injection. Other side effects include allergic reactions. It is in the calcitonin gene-related peptide antagonist class of medications. It was approved for medical use in the United States in 2018, the European Union in 2019 and the UK in 2020. Medical uses Fremanezumab was shown to be effective in adults with four or more attacks per month. Adverse effects The most common adverse effects are reactions at the injection site, which occurred in 43 to 45% of people in studies (as compared to 38% under placebo). Hypersensitivity reactions occurred in fewer than 1% of patients. Interactions Fremanezumab does not interact with other antimigraine drugs such as triptans, ergot alkaloids and analgesics. It is expected to generally have a low potential for interactions because it i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erenumab
Erenumab, sold under the brand name Aimovig, is a medication which targets the calcitonin gene-related peptide receptor (CGRPR) for the prevention of migraine. It is administered by subcutaneous injection. Erenumab, which was developed by Amgen and Novartis, was approved in May 2018, and was the first CGRPR antagonist to be approved by the U.S. Food and Drug Administration. In 2020, it was the 234th most commonly prescribed medication in the United States, with more than 1million prescriptions. Medical uses Erenumab is indicated for the prevention of migraine in adults. Side effects Common side effects are constipation, pruritus, muscle spasms, as well as mild and mostly transient reactions at the injection site. Interactions Erenumab was shown not to interact with ethinylestradiol, norgestimate or the migraine drug sumatriptan. It is expected to generally have a low potential for interactions because it is not metabolized by cytochrome P450 enzymes. Pharmacology Mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Necrotizing Fasciitis
Necrotizing fasciitis (NF), also known as flesh-eating disease, is a bacterial infection that results in the death of parts of the body's soft tissue. It is a severe disease of sudden onset that spreads rapidly. Symptoms usually include red or purple skin in the affected area, severe pain, fever, and vomiting. The most commonly affected areas are the limbs and perineum. Typically, the infection enters the body through a break in the skin such as a cut or burn. Risk factors include poor immune function such as from diabetes or cancer, obesity, alcoholism, intravenous drug use, and peripheral artery disease. It does not typically spread between people. The disease is classified into four types, depending on the infecting organism. Between 55 and 80% of cases involve more than one type of bacteria. Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is involved in up to a third of cases. Medical imaging is often helpful to confirm the diagnosis. Necrotizing fasciitis may b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Botox
Botulinum toxin, or botulinum neurotoxin (BoNT), is a neurotoxic protein produced by the bacterium ''Clostridium botulinum'' and related species. It prevents the release of the neurotransmitter acetylcholine from axon endings at the neuromuscular junction, thus causing flaccid paralysis. The toxin causes the disease botulism. The toxin is also used commercially for medical and cosmetic purposes. The seven main types of botulinum toxin are named types A to G (A, B, C1, C2, D, E, F and G). New types are occasionally found. Types A and B are capable of causing disease in humans, and are also used commercially and medically. Types C–G are less common; types E and F can cause disease in humans, while the other types cause disease in other animals. Botulinum toxins are among the most potent toxins known. Intoxication can occur naturally as a result of either wound or intestinal infection or by ingesting formed toxin in food. The estimated human lethal dose of type A toxin is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Headache
Cluster headache (CH) is a neurological disorder characterized by recurrent severe headaches on one side of the head, typically around the eye(s). There is often accompanying eye watering, nasal congestion, or swelling around the eye on the affected side. These symptoms typically last 15 minutes to 3 hours. Attacks often occur in clusters which typically last for weeks or months and occasionally more than a year. The cause is unknown. Risk factors include a history of exposure to tobacco smoke and a family history of the condition. Exposures which may trigger attacks include alcohol, nitroglycerin, and histamine. They are a primary headache disorder of the trigeminal autonomic cephalalgias type. Diagnosis is based on symptoms. Recommended management includes lifestyle adaptations such as avoiding potential triggers. Treatments for acute attacks include oxygen or a fast-acting triptan. Measures recommended to decrease the frequency of attacks include steroid injections, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galcanezumab
Galcanezumab, sold under the brand name Emgality, is a humanized monoclonal antibody used for the prevention of migraine. It is also used for cluster headaches. Common side effects include pain or redness at the site of injection. Other side effects may include hypersensitivity reactions. A substance called calcitonin gene-related peptide (CGRP) has been shown to be involved in the development of migraine by widening blood vessels in the brain. Galcanezumab is a monoclonal antibody (a type of protein) designed to attach to and block CGRP, thereby helping blood vessels to return to their normal size. This will stop the symptoms of migraine. This drug was developed by Eli Lilly. It was approved for medical use in the United States and in the European Union in 2018, becoming the third calcitonin gene-related peptide (CGRP) inhibitor to do so. When used for migraines it costs about 7,000 per year in the United States . History In September 2018, galcanezumab-gnlm was approved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)