|
Cadaverine
Cadaverine is an organic compound with the formula (CH2)5(NH2)2. Classified as diamine, it is a colorless liquid with an unpleasant odor. It is present in small quantities in living organisms but is often associated with the putrefaction of animal tissue. Production Cadaverine is produced by decarboxylation of lysine.Wolfgang Legrum: ''Riechstoffe, zwischen Gestank und Duft'', Vieweg + Teubner Verlag (2011) S. 65, It can be synthesized by many methods including the hydrogenation of glutaronitrile and the reactions of 1,5-dichloropentane. History Putrescine and cadaverine were first described in 1885 by the Berlin physician Ludwig Brieger (1849–1919). Receptors In zebrafish, the trace amine-associated receptor 13c (or TAAR13c) has been identified as a high-affinity receptor for cadaverine. In humans, molecular modelling and docking experiments have shown that cadaverine fits into the binding pocket of the human TAAR6 and TAAR8. Clinical significance Elevated level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamines
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-diamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skatole
Skatole or 3-methylindole is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary contributor to fecal odor. In low concentrations, it has a flowery smell and is found in several flowers and essential oils, including those of orange blossoms, jasmine, and '' Ziziphus mauritiana''. It is used as a fragrance and fixative in many perfumes and as an aroma compound. Its name derives from the Greek root ''skato-'', meaning feces. Skatole was discovered in 1877 by the German physician Ludwig Brieger (1849–1919). Original: "''Ich habe mich zuerst mit der Untersuchung der flüchtigen Bestandtheile der Excremente aus sauerer Lösung beschäftigt. Es wurden dabei die flüchtigen Fettsäuren: Essigsäure, normale und Isobuttersäure, sowie die aromatischen Substanzen: Phenol, Indol und eine neue dem Indol verwandte Substanz, die ich Skatol nennen werde, erhalten."'' ----''Translation'': "I was occupied ini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Butylamine
''n''-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being ''sec''-butylamine, ''tert''-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Vapors heavier than air and it produces toxic oxides of nitrogen during combustion. Synthesis and reactions It is produced by the reaction of ammonia and alcohols over alumina: :CH3(CH2)3OH + NH3 → CH3(CH2)3NH2 + H2O ''n''-Butylamine is a weak base. The pKa of H3(CH2)3NH3sup>+ is 10.78. ''n''-Butylamine exhibits reactions typical of other simple alkyl amines, i.e., alkylation, acylation, condensation with carbonyls. It forms complexes with metal ions, examples being ''cis''- and ''trans''- tI2(NH2Bu)2 Uses This compound is used as an ingredient in the manufacture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Putrescine
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors. Production Putrescine is produced on an industrial scale by the hydrogenation of succinonitrile. Biotechnological production of putrescine from renewable feedstock has been investigated. A metabolically engineered strain of ''Escherichia coli'' that produces putrescine at high concentrations in glucose mineral salts medium has been described. Biochemistry Spermidine synthase uses putrescine and ''S''-adenosylmethioninamine (decarboxylated ''S''-adenosyl methionine) to produce spermidine. Spermidine in turn is combined with another ''S''-adenosylmethioninamine and gets converted to spermine. Putrescine is synthesized in small quantities by healthy living cells by the action of ornithi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentylamine
1-Aminopentane is an organic compound with the formula CH3(CH2)4NH2. It is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products, and as a flavoring agent.Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke, "Amines, Aliphatic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Pentylamine exhibits reactions typical of other simple alkyl amines, i.e. protonation, alkylation, acylation, condensation with carbonyls. Like other simple aliphatic amines, pentylamine is a weak base: the pKa of H3(CH2)4NH3sup>+ is 10.21. See also * 3-Aminopentane 3-Aminopentane is the organic compound with the formula (CH3CH2)2CHNH2. It is a colorless liquid. It is of interest for producing soluble imides and imines without introducing a chiral center. Safety The LD50 (rat, oral or dermal) for primary al ... References {{DEFAULTSORT:Aminopentane, 1- Alkylamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethylenediamine
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.Robert A. Smiley "Hexamethylenediamine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Synthesis Hexamethylenediamine was first reported by Theodor Curtius. It is produced by the hydrogenation of adiponitrile: :NC(CH2)4CN + 4 H2 → H2N(CH2)6NH2 The hydrogenation is conducted on molten adiponitrile diluted with ammonia, typical catalysts being based on cobalt and iron. The yield is good, but commercially significant side products are generated by virtue of reactivity of partially hydrogenated intermediates. These other products include 1,2-diaminocyclohexane, hexamethyleneimine, and the triamine bis(hexamethylenetriami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Putrescine
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors. Production Putrescine is produced on an industrial scale by the hydrogenation of succinonitrile. Biotechnological production of putrescine from renewable feedstock has been investigated. A metabolically engineered strain of ''Escherichia coli'' that produces putrescine at high concentrations in glucose mineral salts medium has been described. Biochemistry Spermidine synthase uses putrescine and ''S''-adenosylmethioninamine (decarboxylated ''S''-adenosyl methionine) to produce spermidine. Spermidine in turn is combined with another ''S''-adenosylmethioninamine and gets converted to spermine. Putrescine is synthesized in small quantities by healthy living cells by the action of ornithi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
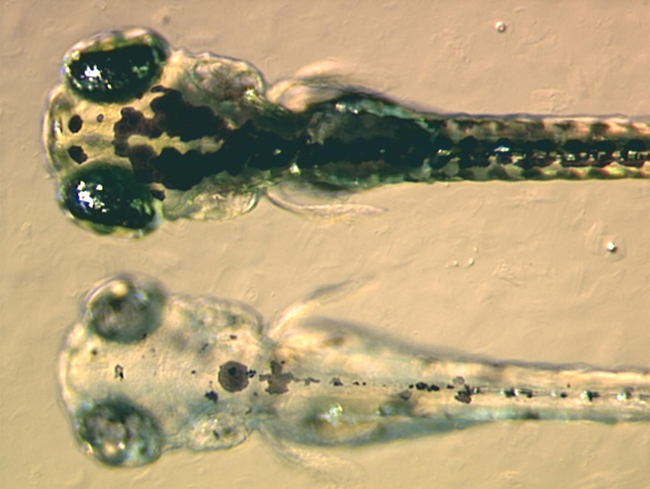
Zebrafish
The zebrafish (''Danio rerio'') is a freshwater fish belonging to the minnow family (Cyprinidae) of the order Cypriniformes. Native to South Asia, it is a popular aquarium fish, frequently sold under the trade name zebra danio (and thus often called a " tropical fish" although both tropical and subtropical). It is also found in private ponds. The zebrafish is an important and widely used vertebrate model organism in scientific research, for example in drug development, in particular pre-clinical development. It is also notable for its regenerative abilities, and has been modified by researchers to produce many transgenic strains. Taxonomy The zebrafish is a derived member of the genus ''Brachydanio'', of the family Cyprinidae. It has a sister-group relationship with '' Danio aesculapii''. Zebrafish are also closely related to the genus '' Devario'', as demonstrated by a phylogenetic tree of close species. Distribution Range The zebrafish is native to fresh wat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acute Toxicity
Acute toxicity describes the adverse effects of a substance that result either from a single exposure or from multiple exposures in a short period of time (usually less than 24 hours). To be described as ''acute'' toxicity, the adverse effects should occur within 14 days of the administration of the substance. Acute toxicity is distinguished from chronic toxicity, which describes the adverse health effects from repeated exposures, often at lower levels, to a substance over a longer time period (months or years). It is widely considered unethical to use humans as test subjects for acute (or chronic) toxicity research. However, some information can be gained from investigating accidental human exposures (e.g., factory accidents). Otherwise, most acute toxicity data comes from animal testing or, more recently, ''in vitro'' testing methods and inference from data on similar substances. Measures of acute toxicity Regulatory values Limits for short-term exposure, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentolinium
Pentolinium is a ganglionic blocking agent which acts as a nicotinic acetylcholine receptor antagonist. Formulated as the pentolinium tartrate salt, it is also known as Ansolysen. It can be used as an antihypertensive drug during surgery or to control hypertensive crises. It works by binding to the acetylcholine receptor of adrenergic nerves and thereby inhibiting the release of noradrenaline and adrenaline. Blocking this receptor leads to smooth muscle relaxation and vasodilation. Route of administration and dose Pentolinium can be given orally (20mg three times a day), injected intramuscularly, or administered intravenously. Use Pentolinium and hexamethonium combined with ''Rauvolfia ''Rauvolfia'' (sometimes spelled ''Rauwolfia'') is a genus of evergreen trees and shrubs, commonly known as devil peppers, in the family Apocynaceae. The genus is named to honor Leonhard Rauwolf. The genus can mainly be found in tropical regi ...'' was reported in 1955 to be effecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |