|
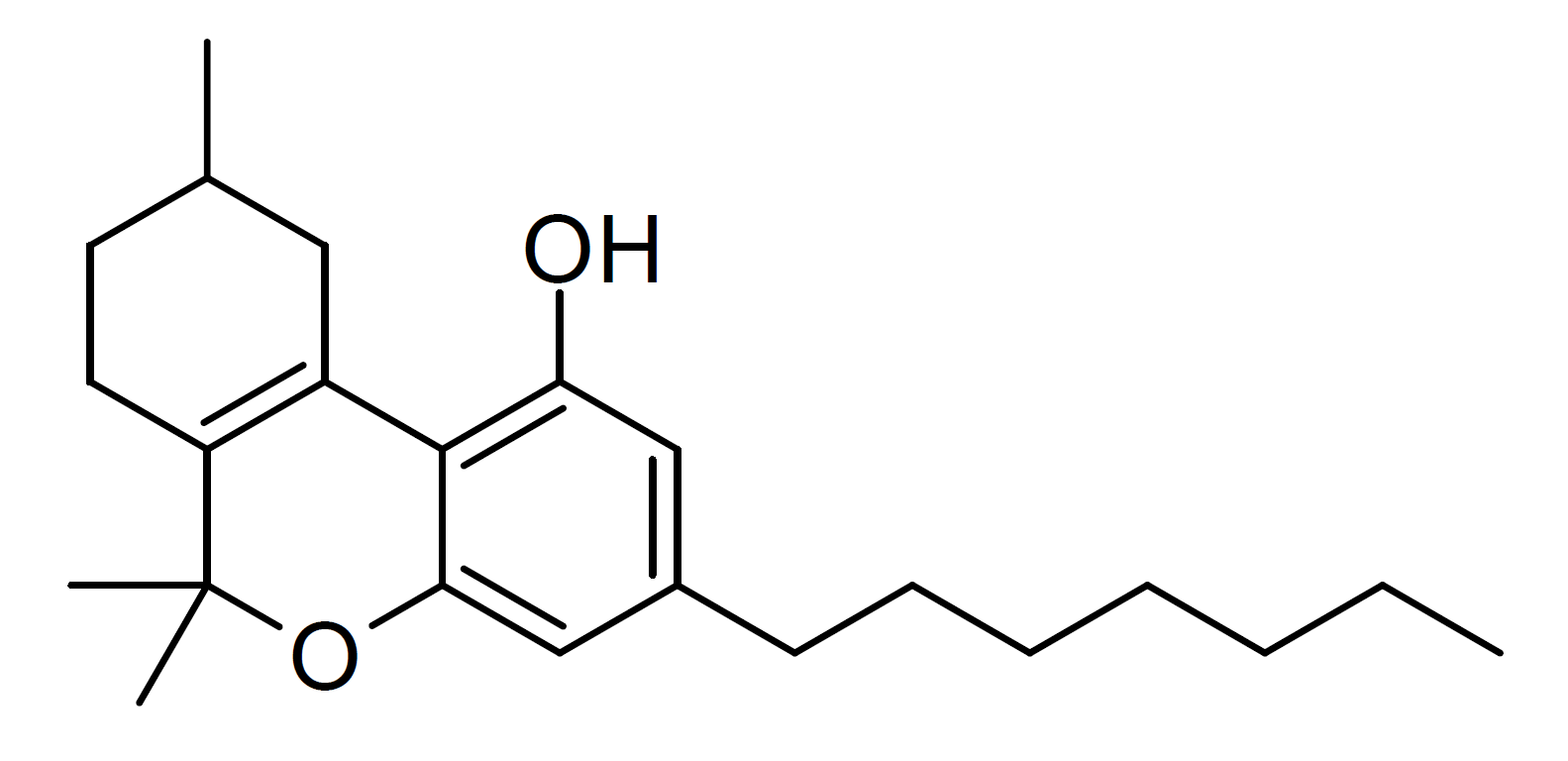
CBD-DMH
Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative. Another closely analogous compound has also been described, with the double bond in the cyclohexene ring shifted to between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabiphorol
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis sativa''. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity approximately 33 times that of Delta-9-THC. Isomers Delta-3-THCP ] The Δ3/Δ6a(10a) isomer Δ3-THCP was synthesised in 1941, and was found to have around the same potency as Delta-3-Tetrahydrocannabinol, Δ3-THC, unlike the hexyl homologue parahexyl which was significantly stronger. Delta-8-THCP The Δ8 isomer is also known as a synthetic cannabinoid under the code name JWH-091, It's unconfirmed whether or not Delta-8-THCP is found naturally in cannabis plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1656
O-1656 is a cannabinoid agonist which was invented by Billy R Martin and Raj K Razdan at Organix Inc in 2002. It is moderately selective for the CB2 receptor with a CB1 receptor affinity of 18 nM and a CB2 receptor affinity of 2 nM. Since it has a cycloheptyl ring attached to the phenol core, it falls outside the definition of a "cyclohexylphenol derivative", but may still be controlled by generic legislation in some jurisdictions. See also * CBD-DMH * CP 55,940 * Cannabidiol * Cannabicyclohexanol * O-1871 O-1871 is a potent cannabinoid agonist which was invented by Billy R Martin and Raj K Razdan at Organix Inc in 2002. It has a CB1 receptor affinity of 2.0nM and a CB2 receptor affinity of 0.3nM. Structurally, O-1871 is a cyclohexylphenol deriv ... References {{cannabinoid-stub Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabidiphorol
The heptyl homologue of cannabidiol was identified as a natural phytocannabinoid and named cannabidiphorol (CBDP) in 2019. It had previously been reported as a synthetic compound, but was not identified as a natural product prior to 2019. References See also * CBD-DMH * Tetrahydrocannabiphorol Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis sativa''. ... {{Cannabinoids Phytocannabinoids 2,6-Dihydroxybiphenyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homolog
In biology, homology is similarity due to shared ancestry between a pair of structures or genes in different taxa. A common example of homologous structures is the forelimbs of vertebrates, where the wings of bats and birds, the arms of primates, the front flippers of whales and the forelegs of four-legged vertebrates like dogs and crocodiles are all derived from the same ancestral tetrapod structure. Evolutionary biology explains homologous structures adapted to different purposes as the result of descent with modification from a common ancestor. The term was first applied to biology in a non-evolutionary context by the anatomist Richard Owen in 1843. Homology was later explained by Charles Darwin's theory of evolution in 1859, but had been observed before this, from Aristotle onwards, and it was explicitly analysed by Pierre Belon in 1555. In developmental biology, organs that developed in the embryo in the same manner and from similar origins, such as from matching primord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-2-CBD-DMH Structure
Increment or incremental may refer to: *Incrementalism, a theory (also used in politics as a synonym for gradualism) *Increment and decrement operators, the operators ++ and -- in computer programming *Incremental computing *Incremental backup, which contain only that portion that has changed since the preceding backup copy. *Increment, chess term for additional time a chess player receives on each move *Incremental games * Increment in rounding See also * * *1+1 (other) 1+1 is a mathematical expression that evaluates to: * 2 (number) (in ordinary arithmetic) * 1 (number) (in Boolean algebra with a notation where '+' denotes a logical disjunction) * 0 (number) (in Boolean algebra with a notation where '+' denotes ' ... {{Disambiguation da:Inkrementel fr:Incrémentation nl:Increment ja:インクリメント pl:Inkrementacja ru:Инкремент sr:Инкремент sv:++ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is a major constituent of temperate Cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four (i.e., THCA, CBDA, CBCA and their common precursor CBGA) have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea. Phytocannabinoids are multi-ring phenolic compounds structurally related to THC, but endocannabinoids are fatty acid derivatives. Nonclassical synthetic cannabinoids (cannabimimetics) include amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NESS-040C5
NESS-040C5 is a potent cannabinoid agonist which was developed for the treatment of glaucoma Glaucoma is a group of eye diseases that result in damage to the optic nerve (or retina) and cause vision loss. The most common type is open-angle (wide angle, chronic simple) glaucoma, in which the drainage angle for aqueous humor, fluid withi .... It has reasonable selectivity for the CB2 receptor subtype, having a CB2 affinity of 0.4nM, and 25x selectivity over the related CB1 receptor. See also * AB-FUBINACA * NESS-0327 * SR-144,528 References {{cannabinoid-stub Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1918
O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan receptors GPR18 and GPR55, that appear to be related to the cannabinoid receptors. O-1918 is used in the study of these receptors, which have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research. See also * Abnormal cannabidiol * Cannabidiol dimethyl ether * CID-16020046 * CID-85469571 * O-1602 * Tetrahydrocannabiorcol Δ9-Tetrahydrocannabiorcol (Δ9-THCC, (C1)-Δ9-THC) is a phytocannabinoid found in ''Cannabis'' pollen. It is a homologue of THC and THCV with the alkyl side chain replaced by a smaller methyl group. Unlike THC and THCV, THCC has negligible affi ... References {{Cannabinoidergics Cannabinoids Cyclohexenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1871
O-1871 is a potent cannabinoid agonist which was invented by Billy R Martin and Raj K Razdan at Organix Inc in 2002. It has a CB1 receptor affinity of 2.0nM and a CB2 receptor affinity of 0.3nM. Structurally, O-1871 is a cyclohexylphenol derivative related to CP 47,497, and so is illegal in some jurisdictions where CP 47,497 and its derivatives are banned. However the 3,3-dimethylcyclohexyl substituent of O-1871 can be replaced by various other groups, producing other potent compounds such as the cycloheptyl derivative O-1656 and the 2-adamantyl derivative O-1660, as well as the corresponding 3,5-dichlorophenyl derivative, which are not cyclohexylphenol derivatives. ] ] See also * CP 55,940 * Cannabidiol * Cannabicyclohexanol Cannabicyclohexanol (CCH, CP 47,497 dimethyloctyl homologue, (C8)-CP 47,497) is a cannabinoid receptor agonist drug, developed by Pfizer in 1979. On 19 January 2009, the University of Freiburg in Germany announced that an analog of CP 47,497 was ... * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KLS-13019
KLS-13019 is a cannabidiol derivative that has been modified on the side chain to improve solubility and tissue penetration properties. It was developed and patented by Neuropathix subsidiary Kannalife and found to be 50x more potent than cannabidiol as a neuroprotective agent, thought to be mediated by modulation of the sodium-calcium exchanger channel. It also had a higher therapeutic index than cannabidiol. Both KLS-13019 and cannabidiol, prevented the development of CIPN, while only KLS-13019 uniquely reversed neuropathic pain from chemotherapy. KLS-13019 binds to fewer biological targets than cannabidiol and KLS-13019 may possess the unique ability to reverse addictive behaviour, an effect not observed with cannabidiol. See also * 7-Hydroxycannabidiol * Abnormal cannabidiol * Cannabidiol dimethyl ether * Delta-6-Cannabidiol * HU-320 * HU-331 * HUF-101 * O-1602 * O-1918 O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HUF-101
4'-Fluorocannabidiol (also known as PECS-101 and 4'-F-CBD, and formerly as HUF-101 and HU-474) is a fluorinated cannabidiol derivative that has more potent anxiolytic, antidepressant, antipsychotic and anti-compulsive activity in mice compared to its parent compound. It was first synthesized in 2016, alongside 10-fluorocannabidiol diacetate and 8,9-dihydro-7-fluorocannabidiol, which showed much weaker activity. Synthesis 4'-Fluorocannabidiol has been synthesized from isolated cannabidiol by putting it in dry dichloromethane and adding 1-fluoropyridinium triflate. See also * 7-Hydroxycannabidiol * 8,9-Dihydrocannabidiol * Abnormal cannabidiol * Cannabinoids * Cannabinoid receptors * HU-331 * KLS-13019 * O-1602 * O-1918 O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan receptors GPR18 and GPR55, that appear to be related to the cannabinoid receptors. O-1918 is used in the study of these receptors, which have been ... Refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-211
Dexanabinol (HU-211 or ETS2101) is a synthetic cannabinoid derivative in development by e-Therapeutics plc. It is the "unnatural" enantiomer of the potent cannabinoid agonist HU-210. Unlike other cannabinoid derivatives, HU-211 does not act as a cannabinoid receptor agonist, but instead has NMDA antagonist effects. It therefore does not produce cannabis-like effects, but is anticonvulsant and neuroprotective, and is widely used in scientific research as well as currently being studied for applications such as treating head injury, stroke, or cancer. It was shown to be safe in clinical trials and is currently undergoing Phase I trials for the treatment of brain cancer and advanced solid tumors. Clinical trials Dexanabinol has been studied in IV administration and oral dosing. e-Therapeutics is evaluating the compound in clinical trials for brain and solid cancers. Phase II studies are planned based on the results of the current trials. A phase 1b study for hepatocellular carci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |