|
Anthraquinone Glycosides
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula . Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite. Synthesis There are several current industrial methods to produce 9,10-anthraquinone: # The oxidation of anthracene. Chromium(VI) is the typical oxidant. # The Friedel-Crafts reaction of benzene and phthalic anhydride in presence of AlCl3. o-Benzoylbenzoic acid is an intermediate. This reaction is useful for produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AlCl3
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color. The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geometry. In contrast, has a more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soda Pulping
Soda pulping is a chemical process for making wood pulp with sodium hydroxide as the cooking chemical. In the ''Soda-AQ'' process, anthraquinone (AQ) may be used as a pulping additive to decrease the carbohydrate degradation. The soda process gives pulp with lower tear strength than other chemical pulping processes ( sulfite process and kraft process), but has still limited use for easily-pulped materials like straw and some hardwoods. History A precursor to the soda pulping process was the paper making process developed by Matthias Koops in 1801 which involved washing wood shavings in limewater, adding soda crystals and then boiling the mixture. Soda pulping was one of the first chemical pulping methods and was invented in 1851 by Burgess (United States) and Watts (England). In France in 1852 Coupier and Mellier patented a soda process based on a 1851 invention the patent of which preceded that of Watt and Burgess, which was filed in 1854. The first mill was started in 1866 in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite Process
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process which produces stronger fibers and is less environmentally costly. History The use of wood to make pulp for paper began with the development of mechanical pulping in the 1840s by Charles Fenerty in Nova ScotiaBurger, Peter 'Charles Fenerty and his Paper Invention''. Toronto: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kraft Process
The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chips with a hot mixture of water, sodium hydroxide (NaOH), and sodium sulfide (Na2S), known as white liquor, that breaks the bonds that link lignin, hemicellulose, and cellulose. The technology entails several steps, both mechanical and chemical. It is the dominant method for producing paper. In some situations, the process has been controversial because kraft plants can release odorous products and in some situations produce substantial liquid wastes. The process name is derived from German word ''Kraft'', meaning "strength" in this context, due to the strength of the kraft paper produced using this process. History A precursor of the Kraft process was used during the Napoleonic Wars in England. The kraft process (so called because o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word "alkali" is derived from Arabic ''al qalīy'' (or ''alkali''), meaning ''the calcined ashes'' (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulp (paper)
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemical or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products. History Before the widely acknowledged invention of papermaking by Cai Lun in China around 105 AD, paper-like writing materials such as papyrus and amate were produced by ancient civilizations using plant materials which were largely unprocessed. Strips of bark or bast material were woven together, beaten into rough sheets, dried, and polished by hand. Pulp used in modern and traditional papermaking is distinguished by the process which produces a finer, more regular slurry of cellulose fibers which are pulled out of solution by a screen and dried to form sheets or rolls. The earliest paper produced in China consisted of bast fibers from the paper mulberr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthrone
Anthrone is a tricyclic aromatic ketone. It is used for a common cellulose assay and in the colorimetric determination of carbohydrates. Derivatives of anthrone are used in pharmacy as laxative. They stimulate the motion of the colon and reduce water reabsorption. Some anthrone derivatives can be extracted from a variety of plants, including ''Rhamnus frangula'', ''Aloe ferox'', '' Rheum officinale'', and ''Cassia senna''. Glycosides of anthrone are also found in high amounts in rhubarb leaves, and alongside concentrated amounts of oxalic acid are the reason for the leaves being inedible. Synthesis and reactions Anthrone can be prepared from anthraquinone by reduction with tin or copper. An alternative synthesis involves cyclization of ''o''-benzylbenzoic acid induced with hydrogen fluoride. Anthrone condenses with glyoxal to give, following dehydrogenation, acedianthrone, a useful octacyclic pigment. Tautomer center, 314px, Tautomeric equilibrium for anthrone. Anthrone is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
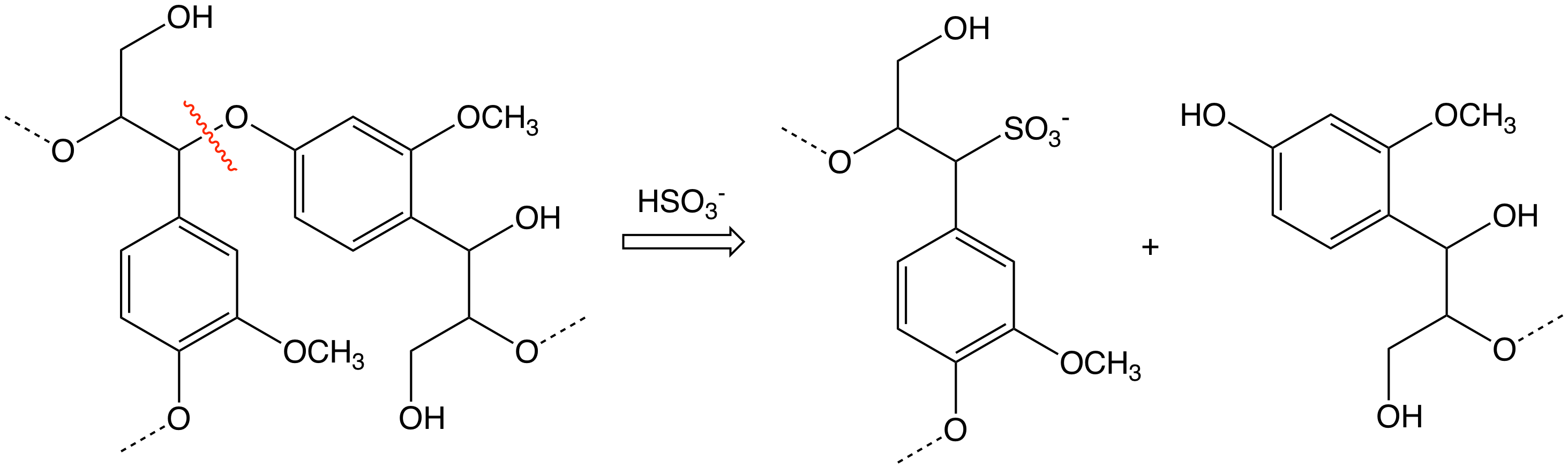
9,10-Dihydroxyanthracene
9,10-Dihydroxyanthracene is an organic compound with the formula (C6H4CHOH)2. It is the hydroquinone form of 9,10-anthraquinone (AQ). It formed when AQ is hydrogenated. It is easily dissolved in alkaline solutions and is often called ''soluble anthraquinone'' (SAQ). In the so-called anthraquinone process, hydrogen peroxide is manufactured as one of the product in the oxygen oxidation of a substituted 9,10-dihydroxyanthracene to its corresponding anthraquinone, such as 2-ethylanthraquinone. See also * Sodium 2-anthraquinonesulfonate Sodium 2-anthraquinonesulfonate (AMS) is a water-soluble anthraquinone derivative. In the laboratory it could be prepared by sulfonation of anthraquinone. Digester additive in papermaking AMS is used as a catalyst in production of alkaline pulpi ..., a water-soluble anthraquinone derivative References {{DEFAULTSORT:Dihydroxyanthracene, 9,10- Anthracenes Phenols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF Societas Europaea, SE () is a German multinational corporation, multinational chemical company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany. The BASF Group comprises subsidiary, subsidiaries and joint ventures in more than 80 countries and operates six integrated production sites and 390 other production sites in Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. At the end of 2019, the company employed 117,628 people, with over 54,000 in Germany. , BASF posted sales of €59.3 billion and income from operations before special items of about €4.5 billion. Between 1990 and 2005, the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam tree (other), balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from the resin (called ''storax'' or ''styrax'' (Latin)) of the Liquidambar styraciflua, American sweetgu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |