|
Aluminium Compounds
Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances.Greenwood and Earnshaw, pp. 222–4 Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency;Greenwood and Earnshaw, pp. 224–7 this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship.Greenwood and Earnshaw, pp. 112–3 However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Bar Surface Etched
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity towards ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Affinity
In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom or compound to combine by chemical reaction with atoms or compounds of unlike composition. History Early theories The idea of ''affinity'' is extremely old. Many attempts have been made at identifying its origins. The majority of such attempts, however, except in a general manner, end in futility since "affinities" lie at the basis of all magic, thereby pre-dating science. Physical chemistry, however, was one of the first branches of science to study and formulate a "theory of affinity". The name ''affinitas'' was first used in the sense of chemical relation by German philosopher Albertus Magnus near the year 1250. Later, those as Robert Boyle, John Mayow, Johann Glauber, Isaac Newton, and Georg Stahl put forward ideas on elective affinity in attem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
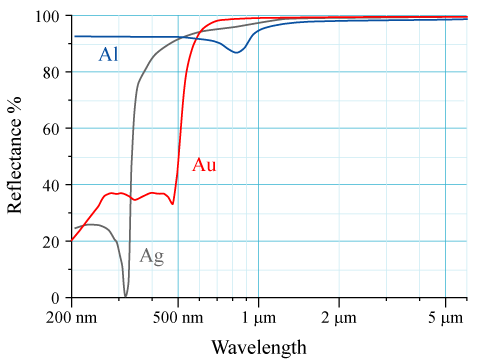
Reflectance
The reflectance of the surface of a material is its effectiveness in Reflection (physics), reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic structure of the material to the electromagnetic field of light, and is in general a function of the frequency, or wavelength, of the light, its polarization, and the angle of incidence (optics), angle of incidence. The dependence of reflectance on the wavelength is called a ''reflectance spectrum'' or ''spectral reflectance curve''. Mathematical definitions Hemispherical reflectance The ''hemispherical reflectance'' of a surface, denoted , is defined as R = \frac, where is the radiant flux ''reflected'' by that surface and is the radiant flux ''received'' by that surface. Spectral hemispherical reflectance The ''spectral hemispherical reflectance in frequency'' and ''spectral hemispherical reflectance in wavelength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver (color)
Silver or metallic gray is a color tone resembling gray that is a representation of the color of polished silver. The visual sensation usually associated with the metal silver is its metallic shine. This cannot be reproduced by a simple solid color because the shiny effect is due to the material's brightness varying with the surface angle to the light source. In addition, there are no mechanism for showing metallic or fluorescent colors on a computer without resorting to rendering software that simulates the action of light on a shiny surface. Consequently, in art and in heraldry, one would typically use a metallic paint that glitters like real silver. A matte grey color could also be used to represent silver. History The first recorded use of ''silver'' as a color name in English was in 1481. In heraldry, the word argent is used, derived from Latin ''argentum'' over Medieval French ''argent''. Silver Displayed at right is the web color silver. Since version 3.2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as '' table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year productio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much large ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butterworth-Heinemann
Butterworth–Heinemann is a British publishing company specialised in professional information and learning materials for higher education and professional training, in printed and electronic forms. It was formed in 1990 by the merger of Heinemann Professional Publishing and Butterworths Scientific, both subsidiaries of Reed International. With its earlier constituent companies, the founding dates back to 1923. It has publishing units in Oxford (UK) and Waltham, Massachusetts (United States). As of 2006, it is an imprint of Elsevier. See also *LexisNexis Butterworths LexisNexis is a part of the RELX corporation that sells data analytics products and various databases that are accessed through online portals, including portals for computer-assisted legal research (CALR), newspaper search, and consumer informa ... References External links * Book publishing companies of the United Kingdom Elsevier imprints {{publish-corp-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane. Volta was the inventor of the voltaic pile, the first electrical battery. In common usage, the word "battery" has come to include a single galvanic cell, but a battery properly consists of multiple cells. History In 1780, Luigi Galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same time to two different parts of a muscle of a frog leg, to close the circuit, the frog's leg contracts. He called this "animal electricity". The frog's leg, as well as being a detector o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amalgam (chemistry)
An amalgam is an alloy of mercury with another metal. It may be a liquid, a soft paste or a solid, depending upon the proportion of mercury. These alloys are formed through metallic bonding, with the electrostatic attractive force of the conduction electrons working to bind all the positively charged metal ions together into a crystal lattice structure. Almost all metals can form amalgams with mercury, the notable exceptions being iron, platinum, tungsten, and tantalum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore. Dentistry has used alloys of mercury with metals such as silver, copper, indium, tin and zinc. Important amalgams Zinc amalgam Zinc amalgam finds use in organic synthesis (e.g., for the Clemmensen reduction). It is the reducing agent in the Jones reductor, used in analytical chemistry. Formerly the zinc plates of dry batteries were amalgamated with a small amount of mercury to pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver and was formerly named hydrargyrum ( ) from the Greek words, ''hydor'' (water) and ''argyros'' (silver). A heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar ( mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide. Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passivation (chemistry)
Passivation, in physical chemistry and engineering, refers to coating a material so it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. In electrochemical treatment of water, passivation reduces the effectiveness of the treatment by increasing the circuit resistance, and active measures are typically used to overcome this effect, the most common being polarity reversal, which results in limited rejection of the fouling layer. When exposed to air, many metals naturally form a hard, relatively inert surface layer, usually a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |