|
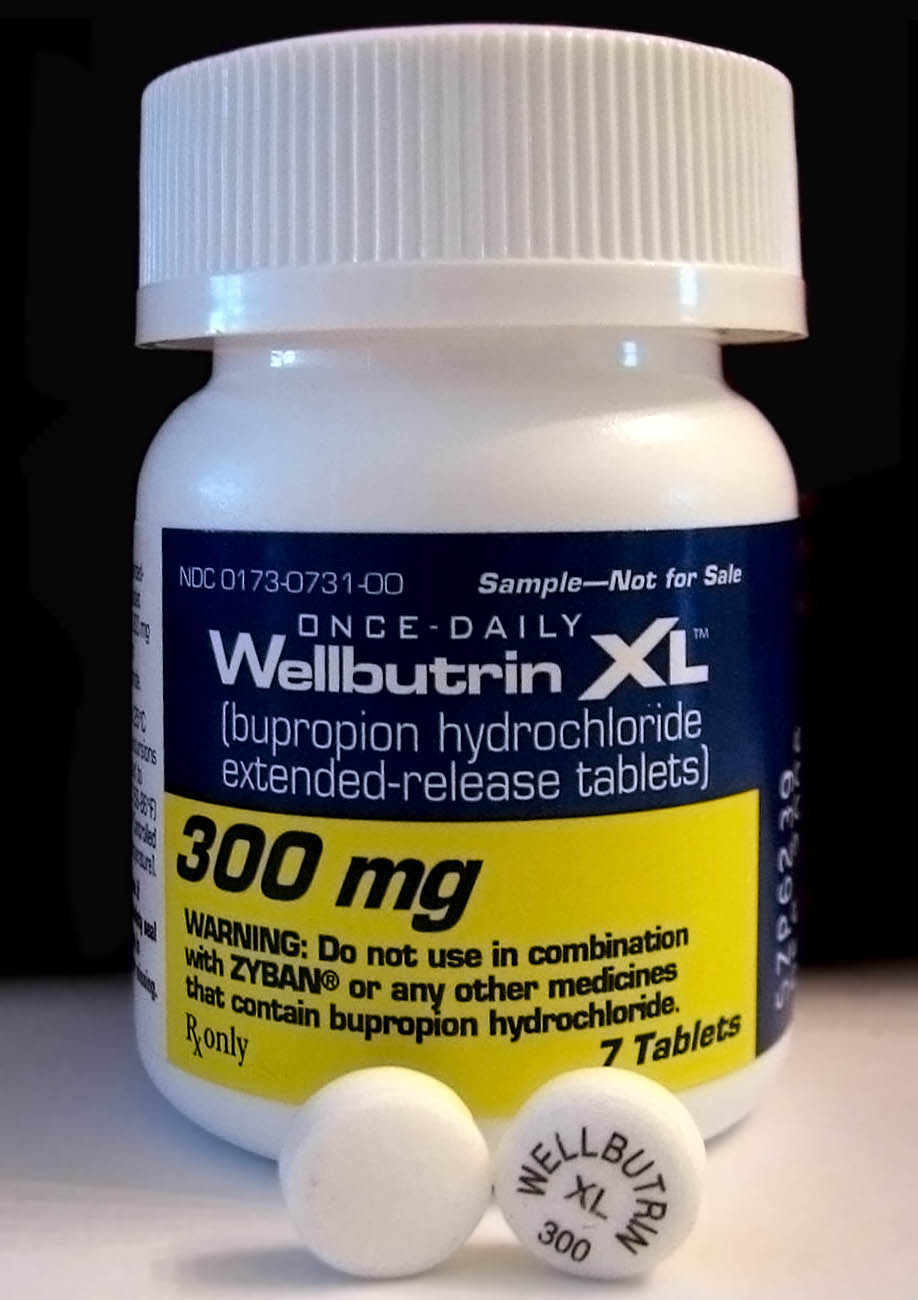
Bupropion
Bupropion, sold under the brand names Wellbutrin and Zyban among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction; it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion does, however, carry a much higher risk of seizure than many other antidepressants and extreme caution must be taken in patients with a history of seizure disorder. Common adverse effects of bupropion with the greatest difference from placebo are dry mouth, nausea, constipation, insomnia, anxiety, tremor, and excessive sweating. Raised blood pressure is notable. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxybupropion
Hydroxybupropion (code name BW 306U), or 6-hydroxybupropion, is the major active metabolite of the antidepressant and smoking cessation drug bupropion. It is formed from bupropion by the liver enzyme CYP2B6 during first-pass metabolism. With oral bupropion treatment, hydroxybupropion is present in plasma at area under the curve concentrations that are as many as 16–20 times greater than those of bupropion itself, demonstrating extensive conversion of bupropion into hydroxybupropion in humans. As such, hydroxybupropion is likely to play a very important role in the effects of oral bupropion, which could accurately be thought of as functioning largely as a prodrug to hydroxybupropion. Other metabolites of bupropion besides hydroxybupropion include threohydrobupropion and erythrohydrobupropion. Pharmacology Pharmacodynamics Compared to bupropion, hydroxybupropion is similar in its potency as a norepinephrine reuptake inhibitor ( IC50 = 1.7 μM), but is substantially weaker a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threohydrobupropion
Threohydrobupropion (developmental code names BW 494, BW A494U) is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a major active metabolite of the antidepressant drug bupropion (Wellbutrin). Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities. Threohydrobupropion exists as two isomers, (1''R'',2''R'')-threohydrobupropion and (1''S'',2''S'')-threohydrobupropion. Other metabolites of bupropion include hydroxybupropion and erythrohydrobupropion. Information on the pharmacological actions of threohydrobupropion is scarce. In any case, it is about 20% as pharmacologically potent as bupropion and in the range of 20 to 50% as potent as bupropion in mouse models of depression. Moreover, threohydrobupropion has been reported to weakly inhibit the reuptake of norepinephrine, dopamine, and serotonin with rat o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythrohydrobupropion
Erythrohydrobupropion (developmental code names BW 287, BW 17U) is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a minor active metabolite of the antidepressant drug bupropion (Wellbutrin). Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities. Erythrohydrobupropion exists as two isomers, (1''R'',2''S'')-erythrohydrobupropion and (1''S'',2''R'')-erythrohydrobupropion. Other metabolites of bupropion include hydroxybupropion and threohydrobupropion. Information on the pharmacological actions of erythrohydrobupropion is scarce. In any case, it is about 20% as pharmacologically potent as bupropion and in the range of 20 to 50% as potent as bupropion in mouse models of depression. It circulates at similar concentrations as bupropion during bupropion therapy. Conversely, two other metabolites, hydroxybupropio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smoking Cessation
Smoking cessation, usually called quitting smoking or stopping smoking, is the process of discontinuing tobacco smoking. Tobacco smoke contains nicotine, which is addictive and can cause dependence. As a result, nicotine withdrawal often makes the process of quitting difficult. Smoking is the leading cause of preventable death and a global public health concern. Tobacco use leads most commonly to diseases affecting the heart and lungs, with smoking being a major risk factor for heart attacks, strokes, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), emphysema, and various types and subtypes of cancers (particularly lung cancer, cancers of the oropharynx, larynx, and mouth, esophageal and pancreatic cancer). Smoking cessation significantly reduces the risk of dying from smoking-related diseases. In the United States, about 70% of smokers would like to quit smoking, and 50% report having made an attempt to do so in the past year. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Cathinone
Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug. List of substituted cathinones The derivatives may be produced by substitutions at four locations of the cathinone molecule: * R1 = hydrogen, or any combination of one or more alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents * R2 = hydrogen or any alkyl group * R3 = hydrogen, any alkyl group, or incorporation in a cyclic structure * R4 = hydrogen, any alkyl group, or incorporation in a cyclic structure The following table displays notable derivatives that have been reported: Legality On 2 April 2010, the Advisory Council on the Misuse of Drugs in the UK announced that a broad structure-based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burroughs Wellcome
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products developed at GSK include several listed in the World Health Organization's List of Essential Medicines, such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selective Serotonin Reuptake Inhibitor
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions. SSRIs increase the extracellular level of the neurotransmitter serotonin by Reuptake inhibitor, limiting its reuptake, reabsorption (reuptake) into the presynaptic cell. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having strong affinity for the serotonin transporter and only weak affinity for the norepinephrine transporter, norepinephrine and dopamine transporters. SSRIs are the most widely prescribed antidepressants in many countries. The efficacy of SSRIs in mild or moderate cases of depression has been disputed and may or may not be outweighed by side effects, especially in adolescent populations. Medical uses The main indication for SSRIs is major depressive disorder; however, they are frequently prescribed for a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Major Depressive Disorder
Major depressive disorder (MDD), also known as clinical depression, is a mental disorder characterized by at least two weeks of pervasive low mood, low self-esteem, and loss of interest or pleasure in normally enjoyable activities. Introduced by a group of US clinicians in the mid-1970s, the term was adopted by the American Psychiatric Association for this symptom cluster under mood disorders in the 1980 version of the '' Diagnostic and Statistical Manual of Mental Disorders'' (DSM-III), and has become widely used since. The diagnosis of major depressive disorder is based on the person's reported experiences, behavior reported by relatives or friends, and a mental status examination. There is no laboratory test for the disorder, but testing may be done to rule out physical conditions that can cause similar symptoms. The most common time of onset is in a person's 20s, with females affected about twice as often as males. The course of the disorder varies widely, from one e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nariman Mehta
Nariman Bomanshaw Mehta (April 20, 1920 – August 22, 2014) was an Indian-born American organic chemist and pharmacologist who designed, synthesized, and patented the organic compound bupropion, marketed under the name Wellbutrin as an antidepressant and smoking cessation aid. Early life and education Mehta was born in Bombay, India into a Parsi Zoroastrian family. He attended St. Xavier's College in Bombay, from where he received Bachelor of Science degrees in chemistry and physics and Bachelor of Arts degrees in English and economics, and a Master of Science degree. In 1939, he and fellow student Kaikhosrov D. Irani, later a noted academic in his own right, wrote and published the book "Textbook of Theoretical and Practical Physics". Mehta won a Tata Scholarship and received a grant from Wendell Willkie. In 1947 Mehta went to the United States, where he earned a PhD in chemistry from The University of Kansas. Mehta's 1952 dissertation was titled ''I. Use of the Hammett eq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Phenethylamine
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH) group via a two-carbon sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms. Many substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., dl-2,5-dimethoxy-4-methylamphetamine DOM), entactogens (e.g., 3,4-methylenedioxyamphet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atypical Antidepressant
An atypical antidepressant is any antidepressant medication that acts in a manner that is different from that of most other antidepressants. Atypical antidepressants include agomelatine, bupropion, iprindole, mianserin, mirtazapine, nefazodone, opipramol, tianeptine, and trazodone. The agents vilazodone and vortioxetine are partly atypical. Typical antidepressants include the , , , and , which act mainly by increasing the levels of the monoamine neurotransmitters serotonin and/or norepinephrine. Among TCAs, trimipramine is an atypical agent in that it appears not to do this. In August 2020, Esketamine (JNJ-54135419) was approved by the U.S. Food and Drug Administration (FDA) for the treatment for treatment-resistant depression with the added indication for the short-term treatment of suicidal thoughts. Buprenorphine/samidorphan Buprenorphine/samidorphan (developmental code name ALKS-5461) is a combination formulation of buprenorphine and samidorphan which is und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Receptor Antagonist
A nicotinic antagonist is a type of anticholinergic drug that inhibits the action of acetylcholine (ACh) at nicotinic acetylcholine receptors. These compounds are mainly used for peripheral muscle paralysis in surgery, the classical agent of this type being tubocurarine,P. Taylor (1990). In ''Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th Ed.'', (A. G. Gilman et al., Eds.), pp. 166-186, New York: Pergamon Press. but some centrally acting compounds such as bupropion, mecamylamine, and 18-methoxycoronaridine block nicotinic acetylcholine receptors in the brain and have been proposed for treating nicotine addiction. *Note: Succinylcholine is a nicotinic agonist An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago .... See neuromuscular blocking agents page for det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |