|
Benzylisoquinoline
Substitution of the Heterocyclic compound, heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline Moiety (chemistry), moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine. Biosynthesis (''S'')-Norcoclaurine (higenamine) has been identified as the central 1-benzyl-tetrahydro-isoquinoline precursor from which numerous complex biosynthetic pathways eventually emerge. These pathways collectively lead to the structurally disparate compounds comprising the broad classification of plant natural products referred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noscapine
Noscapine (also known as Narcotine, Nectodon, Nospen, Anarcotine and (archaic) Opiane) is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae (poppy family). It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects. Medical uses Noscapine is often used as an antitussive medication. A 2012 Dutch guideline, however, does not recommend its use for acute coughing. Side effects *Nausea *Vomiting * Loss of coordination *Hallucinations (auditory and visual) * Loss of sexual drive * Swelling of prostate * Loss of appetite * Dilated pupils *Increased heart rate * Shaking and muscle spasms *Chest pains *Increased alertness *Loss of any sleepiness *Loss of stereoscopic vision Interactions Noscapine can increase the effects of centrally sedating substances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noscapine
Noscapine (also known as Narcotine, Nectodon, Nospen, Anarcotine and (archaic) Opiane) is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae (poppy family). It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects. Medical uses Noscapine is often used as an antitussive medication. A 2012 Dutch guideline, however, does not recommend its use for acute coughing. Side effects *Nausea *Vomiting * Loss of coordination *Hallucinations (auditory and visual) * Loss of sexual drive * Swelling of prostate * Loss of appetite * Dilated pupils *Increased heart rate * Shaking and muscle spasms *Chest pains *Increased alertness *Loss of any sleepiness *Loss of stereoscopic vision Interactions Noscapine can increase the effects of centrally sedating substances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(S)-norcoclaurine Synthase
The enzyme (''S'')-norcoclaurine synthase () catalyzes the chemical reaction : 4-hydroxyphenylacetaldehyde + 4-(2-aminoethyl)benzene-1,2-diol ( Dopamine) \rightleftharpoons (''S'')- norcoclaurine + HO This enzyme belongs to the family of lyases, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is 4-hydroxyphenylacetaldehyde hydro-lyase dding dopamine (''S'')-norcoclaurine-forming''. Other names in common use include (''S'')-norlaudanosoline synthase, and 4-hydroxyphenylacetaldehyde hydro-lyase (adding dopamine). This enzyme participates in benzylisoquinoline alkaloid Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ... biosynthesis. References * * * EC 4.2.1 Enzymes of unknown structure {{4.2-enzyme-s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Higenamine
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including '' Nandina domestica'' (fruit), ''Aconitum carmichaelii'' (root), '' Asarum heterotropioides'', '' Galium divaricatum'' (stem and vine), ''Annona squamosa'', and ''Nelumbo nucifera'' (lotus seeds). Higenamine is found as an ingredient in sports and weight loss dietary supplements sold in the US. The US Food and Drug Administration has received reports of adverse effects from higenamine-containing supplements since 2014, but higenamine's health risks remain poorly understood. Legality Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. Its main use is within food supplements developed for weight management and sports supplements. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies compar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies (''Papaver somniferum''). It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle; by injection under the skin; intravenously; injection into the space around the spinal cord; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mout ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(S)-norcoclaurine
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including ''Nandina domestica'' (fruit), ''Aconitum carmichaelii'' (root), ''Asarum heterotropioides'', '' Galium divaricatum'' (stem and vine), ''Annona squamosa'', and ''Nelumbo nucifera'' (lotus seeds). Higenamine is found as an ingredient in sports and weight loss dietary supplements sold in the US. The US Food and Drug Administration has received reports of adverse effects from higenamine-containing supplements since 2014, but higenamine's health risks remain poorly understood. Legality Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. Its main use is within food supplements developed for weight management and sports supplements. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norcoclaurine
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including ''Nandina domestica'' (fruit), ''Aconitum carmichaelii'' (root), ''Asarum heterotropioides'', '' Galium divaricatum'' (stem and vine), ''Annona squamosa'', and ''Nelumbo nucifera'' (lotus seeds). Higenamine is found as an ingredient in sports and weight loss dietary supplements sold in the US. The US Food and Drug Administration has received reports of adverse effects from higenamine-containing supplements since 2014, but higenamine's health risks remain poorly understood. Legality Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. Its main use is within food supplements developed for weight management and sports supplements. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies (''Papaver somniferum''). It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle; by injection under the skin; intravenously; injection into the space around the spinal cord; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mout ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berberine
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids found in such plants as ''Berberis vulgaris'' (barberry), '' Berberis aristata'' (tree turmeric), '' Mahonia aquifolium'' (Oregon grape), '' Hydrastis canadensis'' (goldenseal), '' Xanthorhiza simplicissima'' (yellowroot), ''Phellodendron amurense'' (Amur cork tree), ''Coptis chinensis'' (Chinese goldthread), ''Tinospora cordifolia'', ''Argemone mexicana'' (prickly poppy), and ''Eschscholzia californica'' (Californian poppy). Berberine is usually found in the roots, rhizomes, stems, and bark. Due to its yellow color, ''Berberis'' species were used to dye wool, leather, and wood. Under ultraviolet light, berberine shows a strong yellow fluorescence, making it useful in histology for staining heparin in mast cells. As a natural dye, berberine has a color index of 75160. Research and adverse effects The safety of using berberine for any condition is not adequately defined by high- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
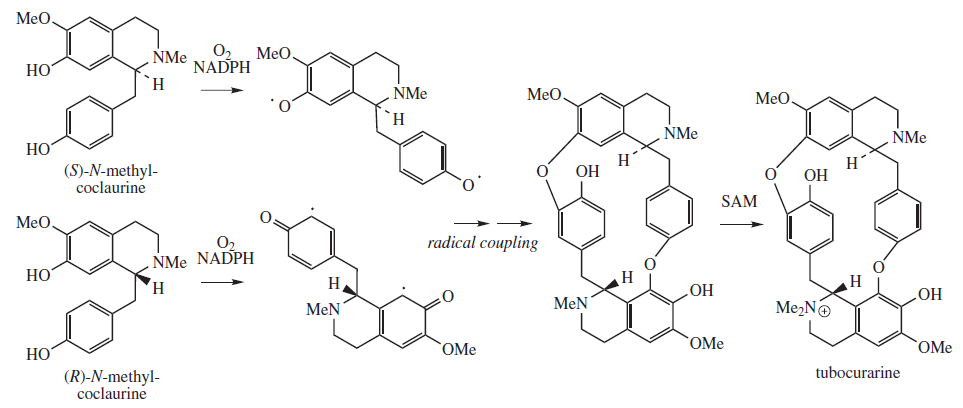
Tubocurarine
Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available. History Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceous South American plant '' Chondrodendron tomentosum'', a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any adverse effects because tubocurarine cannot easily cross mucous membranes. Thus, tubocurarine is effective only if given parenterally, as demons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berberine
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids found in such plants as ''Berberis vulgaris'' (barberry), '' Berberis aristata'' (tree turmeric), '' Mahonia aquifolium'' (Oregon grape), '' Hydrastis canadensis'' (goldenseal), '' Xanthorhiza simplicissima'' (yellowroot), ''Phellodendron amurense'' (Amur cork tree), ''Coptis chinensis'' (Chinese goldthread), ''Tinospora cordifolia'', ''Argemone mexicana'' (prickly poppy), and ''Eschscholzia californica'' (Californian poppy). Berberine is usually found in the roots, rhizomes, stems, and bark. Due to its yellow color, ''Berberis'' species were used to dye wool, leather, and wood. Under ultraviolet light, berberine shows a strong yellow fluorescence, making it useful in histology for staining heparin in mast cells. As a natural dye, berberine has a color index of 75160. Research and adverse effects The safety of using berberine for any condition is not adequately defined by high- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tubocurarine
Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available. History Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceous South American plant '' Chondrodendron tomentosum'', a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any adverse effects because tubocurarine cannot easily cross mucous membranes. Thus, tubocurarine is effective only if given parenterally, as demons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |