|
Bartell Mechanism
The Bartell mechanism is a pseudorotational mechanism similar to the Berry mechanism. It occurs only in molecules with a pentagonal bipyramidal molecular geometry, such as IF7. This mechanism was first predicted by H. B. Bartell. The mechanism exchanges the axial atoms with one pair of the equatorial atoms with an energy requirement of about 2.7 kcal/mol. Similarly to the Berry mechanism in square planar molecules, the symmetry of the intermediary phase of the vibrational mode is "chimeric"LS Bartell, MJ Rothman & A Gavezzotti, 1982, , ''J. Chem. Phys.'' 76:4136-4413.M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ.'' (online), 2005; se, accessed 28 May 2014 of other mechanisms; it displays characteristics of the Berry mechanism, a "lever" mechanism seen in pseud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chemistry (IUPAC) defines a pseudorotation as a " ereoisomerization resulting in a structure that ''appears'' to have been produced by rotation of the entire initial molecule", the result of which is a "product" that is "superposable on the initial one, unless different positions are distinguished by substitution, including isotopic substitution." Well-known examples are the intramolecular isomerization of trigonal bipyramidal compounds by the Berry pseudorotation mechanism, and the out-of-plane motions of carbon atoms exhibited by cyclopentane, leading to the interconversions it experiences between its many possible conformers (envelope, twist). Note, no angular momentum is generated by this motion. In these and related examples, a small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Mechanism
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Ed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentagonal Bipyramidal Molecular Geometry
In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid. A perfect pentagonal bipyramid belongs to the molecular point group ''D5h''. The pentagonal bipyramid is a case where bond angles surrounding an atom are not identical (see also trigonal bipyramidal molecular geometry). This is one of the three common shapes for heptacoordinate transition metal complexes, along with the capped octahedron and the capped trigonal prism. Pentagonal bipyramids are claimed to be promising coordination geometries for lanthanide-based Single-molecule magnets, since (a) they present no extradiagonal Crystal Field terms, therefore minimising spin mixing, and (b) all of their diagonal terms are in first approximation protected from low-energy vibrations, minimising vibronic coupling. Examples * Iodine heptafluoride Iodine heptafluoride, also known as iodine(VII) fluoride or iodine fluoride, is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KK Hii
King Kuok "Mimi" Hii (born 23 December 1969) is a chemist whose fields of research include application of catalysis to organic synthesis. She is the Director of Imperial College London's Centre for Rapid Online Analysis of Reactions (ROAR). Academic career Hii studied for both her BSc and PhD at The University of Leeds investigating multidentate ligand metal complexes under the supervision of Bernard L. Shaw. Postdoctoral studies at Oxford University in John M. Brown's group were followed by independent research at University of Leeds, Kings College London and in Imperial College London. In 2016 she was awarded a Professorship in Catalysis and in 2018 became the Director of the Centre for Rapid Online Analysis of Reactions. Research interests Hii has investigated catalysis during her research career. In Oxford, she characterised intermediates of the Heck reaction. Hii also studied this reaction at King's College London. Hii has continued with this field of study producing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seesaw (chemistry)
Disphenoidal or seesaw (also known as sawhorse) is a type of molecular geometry where there are four bonds to a central atom with overall C2v molecular symmetry. The name "seesaw" comes from the observation that it looks like a playground seesaw. Most commonly, four bonds to a central atom result in tetrahedral or, less commonly, square planar geometry. The seesaw geometry occurs when a molecule has a steric number of 5, with the central atom being bonded to 4 other atoms and 1 lone pair (AX4E1 in AXE notation). An atom bonded to 5 other atoms (and no lone pairs) forms a trigonal bipyramid with two axial and three equatorial positions, but in the seesaw geometry one of the atoms is replaced by a lone pair of electrons, which is always in an equatorial position. This is true because the lone pair occupies more space near the central atom (A) than does a bonding pair of electrons. An equatorial lone pair is repelled by only two bonding pairs at 90°, whereas a hypothetical axial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
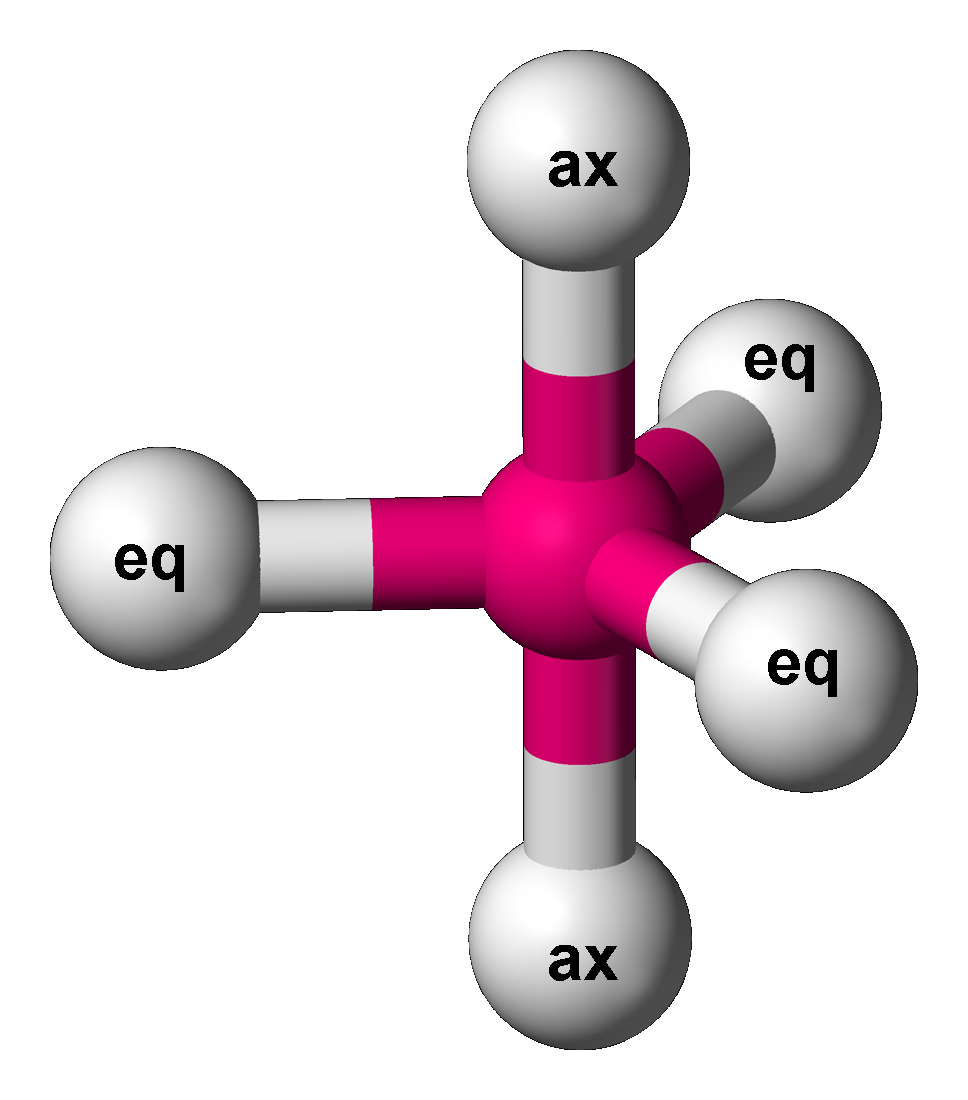
Trigonal Bipyramidal
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chemistry (IUPAC) defines a pseudorotation as a " ereoisomerization resulting in a structure that ''appears'' to have been produced by rotation of the entire initial molecule", the result of which is a "product" that is "superposable on the initial one, unless different positions are distinguished by substitution, including isotopic substitution." Well-known examples are the intramolecular isomerization of trigonal bipyramidal compounds by the Berry pseudorotation mechanism, and the out-of-plane motions of carbon atoms exhibited by cyclopentane, leading to the interconversions it experiences between its many possible conformers (envelope, twist). Note, no angular momentum is generated by this motion. In these and related examples, a small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bailar Twist
The Bailar twist is a mechanism proposed for the racemization of octahedral complexes containing three bidentate chelate rings. Such complexes typically adopt an octahedral molecular geometry, in which case they possess helical chirality. One pathway by which these compounds can racemize is via the formation of a trigonal prismatic intermediate with D3h point group symmetry. This pathway is named in honor of John C. Bailar, Jr., an inorganic chemist who investigated this process.{{cite journal , author = A. Rodger, B. F. G. Johnson , title = Which is more likely: the Ray–Dutt twist or the Bailar twist? , journal = Inorganic Chemistry , year = 1988 , volume = 27 , issue = 18 , pages = 3061–3062 , doi =10.1021/ic00291a001 An alternative pathway is called the Ray–Dutt twist. See also * Pseudorotation * Bartell mechanism * Berry mechanism * Ray–Dutt twist * Fluxional molecule In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ray–Dutt Twist
The Ray–Dutt twist is a mechanism proposed for the racemization of octahedral complexes containing three bidentate chelate rings. Such complexes typically adopt an octahedral molecular geometry in their ground states, in which case they possess helical chirality. The pathway entails formation of an intermediate of C2v point group symmetry. An alternative pathway that also does not break any metal-ligand bonds is called the Bailar twist. Both of these mechanism product complexes wherein the ligating atoms (X in the scheme) are arranged in an approximate trigonal prism. This pathway is called the Ray–Dutt twist in honor of Priyadaranjan Ray (not Prafulla Chandra Ray) and N. K. Dutt, inorganic chemists at the Indian Association for the Cultivation of Science abbr. ''IACS'' who proposed this process. See also * Pseudorotation * Bailar twist * Bartell mechanism * Berry mechanism * Fluxional molecule In chemistry and molecular physics, fluxional (or non-rigid) molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluxional Molecule
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods. Spectroscopic studies Many organometallic compounds exhibit fluxionality. Fluxionality is however pervasive. NMR spectroscopy Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates compara ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |