|
Bromobenzyl Cyanide
Bromobenzyl cyanide (BBC) is an obsolete lachrymatory agent introduced in World War I by the Allied Powers. When implemented in World War I, it revolutionized the use of tear agents due to their extreme potency. BBC is toxic like chlorine gas.AMOS A. FRIES and CLARENCE J. WEST. CHEMICAL WARFARE - First Edition. p 142-143 See also *Chloroacetophenone *CR gas *CS gas *Lachrymatory agent Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In ad ... References External links * * Benzene derivatives Lachrymatory agents Nitriles Organobromides {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lachrymatory Agent
Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray (OC gas), PAVA spray (nonivamide), CS gas, CR gas, CN gas (phenacyl chloride), bromoacetone, xylyl bromide and Mace (a branded mixture). While lachrymatory agents are commonly deployed for riot control by law enforcement and military personnel, its use in warfare is prohibited by various international treaties.E.g. the Geneva Protocol of 1925 prohibited the use of "asphyxiating gas, or any other kind of gas, liquids, substances or similar materials". During World War I, increasingly toxic and deadly lachrymatory agents were used. The short and long-term effec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fighting occurring throughout Europe, the Middle East, Africa, the Pacific, and parts of Asia. An estimated 9 million soldiers were killed in combat, plus another 23 million wounded, while 5 million civilians died as a result of military action, hunger, and disease. Millions more died in genocides within the Ottoman Empire and in the 1918 influenza pandemic, which was exacerbated by the movement of combatants during the war. Prior to 1914, the European great powers were divided between the Triple Entente (comprising France, Russia, and Britain) and the Triple Alliance (containing Germany, Austria-Hungary, and Italy). Tensions in the Balkans came to a head on 28 June 1914, following the assassination of Archduke Franz Ferdin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allies Of World War I
The Allies of World War I, Entente Powers, or Allied Powers were a coalition of countries led by France, the United Kingdom, Russia, Italy, Japan, and the United States against the Central Powers of Germany, Austria-Hungary, the Ottoman Empire, Bulgaria, and their colonies during the First World War (1914–1918). By the end of the first decade of the 20th century, the major European powers were divided between the Triple Entente and the Triple Alliance. The Triple Entente was made up of France, Britain, and Russia. The Triple Alliance was originally composed of Germany, Austria–Hungary, and Italy, but Italy remained neutral in 1914. As the war progressed, each coalition added new members. Japan joined the Entente in 1914 and after proclaiming its neutrality at the beginning of the war, Italy also joined the Entente in 1915. The term "Allies" became more widely used than "Entente", although France, Britain, Russia, and Italy were also referred to as the Quadruple Entente ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetophenone
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Preparation Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: Riot control agent It was investigated, but not used, during the First and Second World Wars. Because of its significantly greater toxicity, it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “ Mace” or tear gas, its use is falling as pepper spray both works and disperses mor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CR Gas
CR gas or dibenzoxazepine (chemical name dibenz 'b'',''f''1,4]oxazepine, is an incapacitating agent and a lachrymatory agent. CR was developed by the British Ministry of Defence as a riot control agent in the late 1950s and early 1960s. A report from the Porton Down laboratories described exposure as "like being thrown blindfolded into a bed of stinging nettles", and it earned the nickname "firegas". In its effects, the CR gas is very similar (but twice as potent) to o-chlorobenzylidene malononitrile CS gas, even though there is little structural resemblance between the two. For example, 2 mg of dry CR cause skin redness in 10 min, 5 mg cause burning and erythremia, 20 mg- strong pain. Water usually amplifies the pain effect of CR on skin. CR aerosols cause irritation as 0.0002 mg/L, which becomes intolerable at 0.003 mg/L. The lethal dose of CR through air inhalation is LD50 = 350 mg·min/L. Physical properties and deployment CR is a pale yellow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CS Gas
The compound 2-chlorobenzalmalononitrile (also called ''o''-chlorobenzylidene malononitrile; chemical formula: C10H5ClN2), a cyanocarbon, is the defining component of tear gas commonly referred to as CS gas, which is used as a riot control agent. Exposure causes a burning sensation and tearing of the eyes to the extent that the subject cannot keep their eyes open, and a burning irritation of the mucous membranes of the nose, mouth and throat, resulting in profuse coughing, nasal mucus discharge, disorientation, and difficulty breathing, partially incapacitating the subject. CS gas is an aerosol of a volatile solvent (a substance that dissolves other active substances and that easily evaporates) and 2-chlorobenzalmalononitrile, which is a solid compound at room temperature. CS gas is generally accepted as being non-lethal. It was first synthesized by two Americans, Ben Corson and Roger Stoughton, at Middlebury College in 1928, and the chemical's name is derived from the firs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lachrymatory Agent
Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray (OC gas), PAVA spray (nonivamide), CS gas, CR gas, CN gas (phenacyl chloride), bromoacetone, xylyl bromide and Mace (a branded mixture). While lachrymatory agents are commonly deployed for riot control by law enforcement and military personnel, its use in warfare is prohibited by various international treaties.E.g. the Geneva Protocol of 1925 prohibited the use of "asphyxiating gas, or any other kind of gas, liquids, substances or similar materials". During World War I, increasingly toxic and deadly lachrymatory agents were used. The short and long-term effec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene Derivatives
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lachrymatory Agents
Lachrymatory or lacrymatory may refer to: * Something that has the effect of ''lachrymation'', causing the secretion of tears * Tear gas Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In ad ..., known formally as a ''lachrymatory agent'' or ''lachrymator'' * A lacrymatory, a small vessel of terracotta or glass found in Roman and late Greek tombs, thought to have been used to collect the tears of mourners at funerals {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geometry is linear in nitriles, reflecting the sp hybridization of the triply bonded carbon. The C−N distance is short at 1.16 Å, consistent with a triple bond. Nitriles a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)
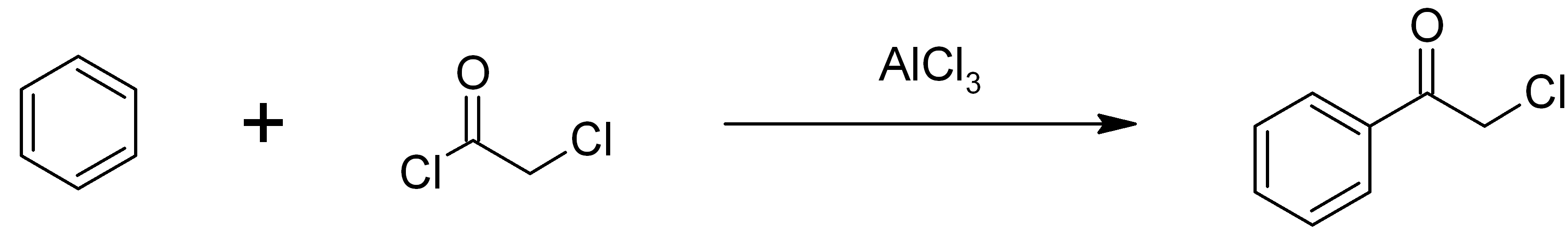
2.png)
