|
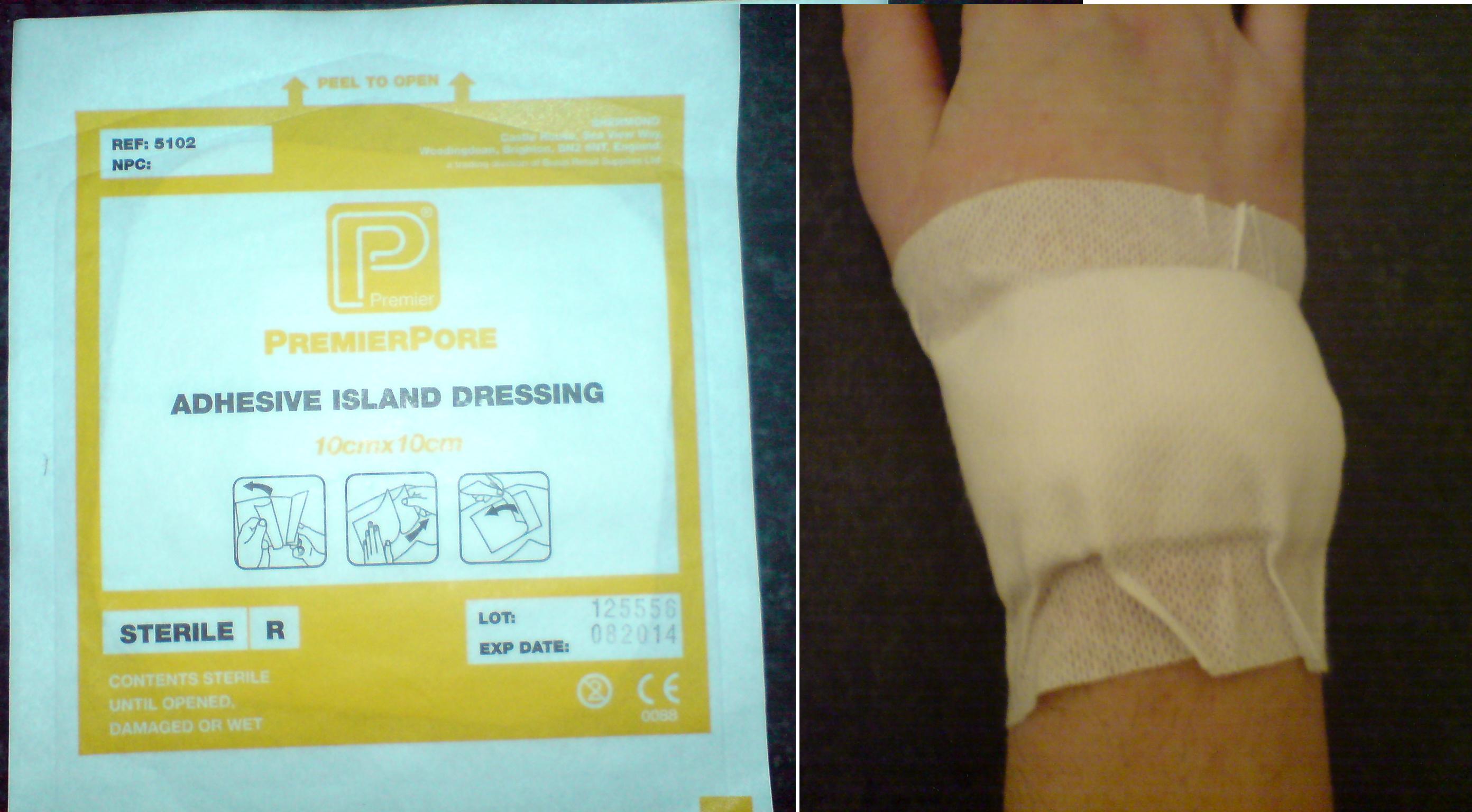
Boracic Lint
Boracic lint () is a type of medical dressing made from surgical lint that is soaked in a hot, saturated solution of boracic acid and glycerine and then left to dry. It has been in use since at least the 19th century, but is now less commonly used. When in use, boracic lint proved to be very valuable in the treatment of leg ulcers. The term "boracic" is also used as Cockney rhyming slang Rhyming slang is a form of slang word construction in the English language. It is especially prevalent among Cockneys in England, and was first used in the early 19th century in the East End of London; hence its alternative name, Cockney rhymin ... for having no money: "boracic lint" → " skint". References First aid {{treatment-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Dressing
A dressing is a sterile pad or compress applied to a wound to promote healing and protect the wound from further harm. A dressing is designed to be in direct contact with the wound, as distinguished from a bandage, which is most often used to hold a dressing in place. Many modern dressings are self-adhesive. Medical uses A dressing can have a number of purposes, depending on the type, severity and position of the wound, although all purposes are focused on promoting recovery and protecting from further harm. Key purposes of a dressing are: * Stop bleeding – to help to seal the wound to expedite the clotting process; * Protection from infection – to defend the wound against germs and mechanical damage; * Absorb exudate – to soak up blood, plasma, and other fluids exuded from the wound, containing it/them in one place and preventing maceration; * Ease pain – either by a medicated analgesic effect, compression or simply preventing pain from further trauma; * Debride the w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lint (material)
Lint is the common name for visible accumulations of textile fibers and other materials, usually found on and around clothing. Certain materials used in the manufacture of clothing, such as cotton, linen, and wool, contain numerous, very short fibers bundled together. During the course of normal wear, these fibers may either detach or be jostled out of the weave of which they are part. This is the reason why heavily-used articles such as shirts and towels become thin over time and why such particles accumulate in the lint screen of a clothes dryer. Because of their high surface area to weight ratio, static cling causes fibers that have detached from an article of clothing to continue to stick to one another and to that article or other surfaces with which they come in contact. Other small fibers or particles also accumulate with these clothing fibers, including human and animal hair and skin cells, plant fibers, and pollen, dust, and microorganisms. The etymology of the moder ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Solution
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxoacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However boric acid and borates have been used since the time of the ancient Greeks for cleaning, preserving food, and other activities. Molecular a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cockney Rhyming Slang
Rhyming slang is a form of slang word construction in the English language. It is especially prevalent among Cockneys in England, and was first used in the early 19th century in the East End of London; hence its alternative name, Cockney rhyming slang. In the US, especially the criminal underworld of the West Coast between 1880 and 1920, rhyming slang has sometimes been known as Australian slang. The construction of rhyming slang involves replacing a common word with a phrase of two or more words, the last of which rhymes with the original word; then, in almost all cases, omitting, from the end of the phrase, the secondary rhyming word (which is thereafter implied), Bryson, a humourist, states that there is a special name given to this omission: "the word that rhymes is almost always dropped... There's a technical term for this process as well: hemiteleia". Given that this is a genus of plant species, and appears in no readily available sources as a linguistic term, it is unclea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |