|
Bog Ore
Bog iron is a form of impure iron deposit that develops in bogs or swamps by the chemical or biochemical oxidation of iron carried in solution. In general, bog ores consist primarily of iron oxyhydroxides, commonly goethite (FeO(OH)). Iron-bearing groundwater typically emerges as a spring and the iron in it forms ferric hydroxide upon encountering the oxidizing environment of the surface. Bog ore often combines goethite, magnetite, and vugs or stained quartz. Oxidation may occur through enzyme catalysis by iron bacteria. It is not clear whether the magnetite precipitates upon the first contact with oxygen, then oxidizes to ferric compounds, or whether the ferric compounds are reduced when exposed to anoxic conditions upon burial beneath the sediment surface and reoxidized upon exhumation at the surface. Bog iron, like other hydrous iron oxides, has a specific affinity for heavy metals. This affinity combined with the porous structure and high specific surface area of bog iro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limonite Bog Iron Cm02
Limonite () is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·H2O, although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Limonite is one of the three principal iron ores, the others being hematite and magnetite, and has been mined for the production of iron since at least 2500 BP. Names Limonite is named for the Greek word λειμών (/leː.mɔ̌ːn/), meaning "wet meadow", or λίμνη (/lím.nɛː/), meaning “marshy lake” as an allusion to its occurrence as '' bog iron ore'' in meadows and marshes. In its brown form it is sometimes called brown hematite or brown iron ore. Characteristics Limonite is relatively dense with a specific gravity varying from 2.7 to 4.3.Northrop, Stuart A. (1959) "Limonite" ''Minerals of New Mexico'' (revised edition) University of New Mexico Press, Albuquerque, New Mexico, pp. 329– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pore Structure
Pore structure is a common term employed to characterize the porosity, pore size, pore size distribution, and pore morphology (such as pore shape, surface roughness, and tortuosity of pore channels) of a porous medium. Pores are the openings in the surfaces impermeable porous matrix which gases, liquids, or even foreign microscopic particles can inhabit them. The pore structure and fluid flow in porous media are intimately related. With micronanoscale pore radii, complex connectivity, and significant heterogeneity, the complexity of the pore structure affects the hydraulic conductivity and retention capacity of these fluids. The intrinsic permeability is the attribute primarily influenced by the pore structure, and the fundamental physical factors governing fluid flow and distribution are the grain surface-to-volume ratio and grain shape. The idea that the pore space is made up of a network of channels through which fluid can flow is particularly helpful. Pore openings are t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schwertmannite
Schwertmannite is an iron-oxyhydroxysulfate mineral with an ideal chemical formula of or . It is an opaque tetragonal mineral typically occurring as brownish yellow encrustations. It has a Mohs hardness of 2.5 - 3.5 and a specific gravity of 3.77 - 3.99. It was first described for an occurrence in Finland in 1994 and named for Udo Schwertmann (born 1927) soil scientist, Technical University of Munich, Munich, Germany. Schwertmannite (with a distinct "pin cushion" morphology) commonly forms in iron-rich, acid sulfate waters in the pH-range of 2 - 4. The mineral was first recognised officially as a new mineral from a natural acid-sulfate spring occurrence at Pyhäsalmi, Finland. However, it is more commonly reported as an orange precipitate in streams and lakes affected by acid mine drainage. Schwertmannite is also known to be central to iron-sulfur geochemistry in acid sulfate soil Acid sulfate soils are naturally occurring soils, sediments or organic substrates (e.g. peat) tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . It has the same crystal structure as corundum () and ilmenite (). With this it forms a complete solid solution at temperatures above . Hematite naturally occurs in black to steel or silver-gray, brown to reddish-brown, or red colors. It is mined as an important ore mineral of iron. It is electrically conductive. Hematite varieties include ''kidney ore'', ''martite'' (pseudomorphs after magnetite), ''iron rose'' and ''specularite'' (specular hematite). While these forms vary, they all have a rust-red streak. Hematite is not only harder than pure iron, but also much more brittle. Maghemite is a polymorph of hematite (γ-) with the same chemical formula, but with a spinel structure like magnetite. Large deposits of hematite are found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiobacillus Thiooxidans
''Acidithiobacillus thiooxidans'', formerly known as ''Thiobacillus thiooxidans'' until its reclassification into the newly designated genus ''Acidithiobacillus'' of the Acidithiobacillia subclass of Pseudomonadota, is a Gram-negative, rod-shaped bacterium that uses sulfur as its primary energy source. It is mesophilic, with a temperature optimum of 28 °C. This bacterium is commonly found in soil, sewer pipes, and cave biofilms called snottites. ''A. thiooxidans'' is used in the mining technique known as bioleaching, where metals are extracted from their ores through the action of microbes. Morphology ''A. thiooxidans'' is a Gram-negative, rod-shaped bacterium with rounded ends that occurs in nature either as singlecells, as is the most common case, or sometimes in pairs, but rarely in triplets. Its motility is due to a polar flagellum. It is an obligate acidophile with an optimal pH less than 4.0, but it also qualifies as an obligate aerobe and chemolithotroph. Describ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiobacillus Ferrooxidans
''Acidithiobacillus'' is a genus of the ''Acidithiobacillia'' in the "Pseudomonadota". The genus includes acidophilic organisms capable of iron and/or sulfur oxidation. Like all ''"Pseudomonadota"'', ''Acidithiobacillus'' spp. are Gram-negative. They are also important generators of acid mine drainage, which is a major environmental problem around the world in mining. Genus ''Acidithiobacillus'' ''Acidithiobacillus'' are acidophilic obligate autotrophs (''Acidithiobacillus caldus'' can also grow mixotrophically) that use elementary sulfur, tetrathionate and ferrous iron as electron donors. They assimilate carbon from carbon dioxide using the transaldolase variant of the Calvin-Benson-Bassham cycle. The genus comprises motile, rod-shaped cells that can be isolated from low pH environments including low pH microenvironments on otherwise neutral mineral grains. Phylogeny The order Acidithiobacillales (i.e. ''Thermithiobacillus'') were formerly members of the ''Gammaproteobacteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-oxidizing Bacteria
Iron-oxidizing bacteria are chemotrophic bacteria that derive energy by oxidation, oxidizing dissolved ferrous iron#Bioinorganic compounds, iron. They are known to grow and proliferate in waters containing iron concentrations as low as 0.1 mg/L. However, at least 0.3 ppm of dissolved oxygen is needed to carry out the oxidation. Iron is a very important Chemical element, element required by living Organism, organisms to carry out numerous Metabolism, metabolic reactions such as the formation of Protein, proteins involved in Biochemistry, biochemical reactions. Examples of these proteins include iron–sulfur proteins, hemoglobin, and coordination complexes. Iron has a widespread distribution globally and is considered one of the most abundant in the Earth's crust, soil, and sediments. Iron is a trace element in Marine habitats, marine environments. Its role in the metabolism of some chemolithotrophs is probably very ancient. As Liebig's law of the minimum notes, the essential eleme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen Saturation
Oxygen saturation (symbol SO2) is a relative measure of the concentration of oxygen that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium at the given temperature. It can be measured with a dissolved oxygen probe such as an oxygen sensor or an optode in liquid media, usually water. The standard unit of oxygen saturation is percent (%). Oxygen saturation can be measured regionally and noninvasively. Arterial oxygen saturation (SaO2) is commonly measured using pulse oximetry. Tissue saturation at peripheral scale can be measured using NIRS. This technique can be applied on both muscle and brain. In medicine In medicine, oxygen saturation refers to ''oxygenation'', or when oxygen molecules () enter the tissues of the body. In this case blood is oxygenated in the lungs, where oxygen molecules travel from the air into the blood. Oxygen saturation (() sats) measures the percentage of hemoglobin binding s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Bearing Water In A Spring
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), and is typically associated with the corrosion of refined iron. Given sufficient time, any iron mass, in the presence of water and oxygen, could eventually convert entirely to rust. Surface rust is commonly flaky and friable, and provides no passivational protection to the underlying iron, unlike the formation of patina on copper surfaces. ''Rusting'' is the common term for corrosion of elemental iron and its alloys such as steel. Many other metals undergo similar corrosion, but the resulting oxides are not commonly called "rust". Several forms of rust are distinguishable both visually and by spectroscopy, and form under different circumstances. Other forms of rust include the result of reactions between iron and chloride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
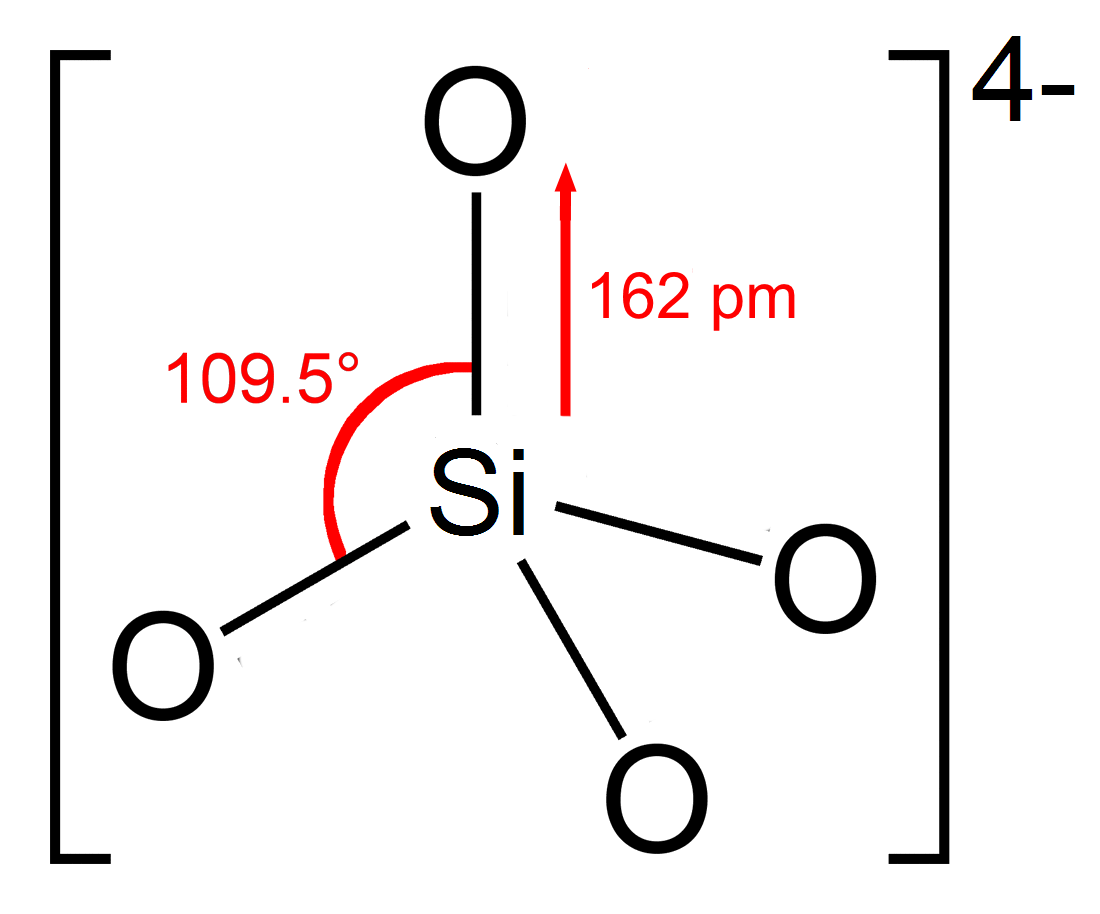
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedron whose corner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |