|
Beta-alumina Solid Electrolyte
Beta-alumina solid electrolyte (BASE) is a fast ion conductor material used as a membrane in several types of molten salt electrochemical cell. Currently there is no known substitute available. β-Alumina exhibits an unusual layered crystal structure which enables very fast ion transport. β-Alumina is not an isomorphic form of aluminium oxide (Al2O3), but a sodium polyaluminate. It is a hard polycrystalline ceramic, which, when prepared as an electrolyte, is complexed with a mobile ion, such as Na+, K+, Li+, Ag+, H+, Pb2+, Sr2+ or Ba2+ depending on the application. β-Alumina is a good conductor of its mobile ion yet allows no non-ionic (i.e., electronic) conductivity. The crystal structure of the β-alumina provides an essential rigid framework with channels along which the ionic species of the solid can migrate. Ion transport involves hopping from site to site along these channels. Since the 1970's this technology has been thoroughly developed, resulting in interesting a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Ion Conductor
In materials science, fast ion conductors are solid conductors with highly mobile ions. These materials are important in the area of solid state ionics, and are also known as solid electrolytes and superionic conductors. These materials are useful in batteries and various sensors. Fast ion conductors are used primarily in solid oxide fuel cells. As solid electrolytes they allow the movement of ions without the need for a liquid or soft membrane separating the electrodes. The phenomenon relies on the hopping of ions through an otherwise rigid crystal structure. Mechanism Fast ion conductors are intermediate in nature between crystalline solids which possess a regular structure with immobile ions, and liquid electrolytes which have no regular structure and fully mobile ions. Solid electrolytes find use in all solid-state supercapacitors, batteries, and fuel cells, and in various kinds of chemical sensors. Classification In solid electrolytes (glasses or crystals), the ionic condu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Grove in 1838. The first commercial use of fuel cells came more than a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for satellites and space capsules. Since then, fuel cells have b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Ion Conductor
In materials science, fast ion conductors are solid conductors with highly mobile ions. These materials are important in the area of solid state ionics, and are also known as solid electrolytes and superionic conductors. These materials are useful in batteries and various sensors. Fast ion conductors are used primarily in solid oxide fuel cells. As solid electrolytes they allow the movement of ions without the need for a liquid or soft membrane separating the electrodes. The phenomenon relies on the hopping of ions through an otherwise rigid crystal structure. Mechanism Fast ion conductors are intermediate in nature between crystalline solids which possess a regular structure with immobile ions, and liquid electrolytes which have no regular structure and fully mobile ions. Solid electrolytes find use in all solid-state supercapacitors, batteries, and fuel cells, and in various kinds of chemical sensors. Classification In solid electrolytes (glasses or crystals), the ionic condu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
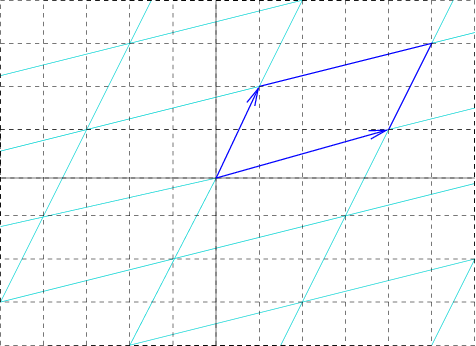
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a three-dimensional figure with six faces which are rhombus, rhombi. It is a special case of a parallelepiped where all edges are the same length. It can be used to define the rhombohedral lattice system, a Honeycomb (geometry), honeycomb with rhombohedral cells. A cube is a special case of a rhombohedron with all sides square. In general a ''rhombohedron'' can have up to three types of rhombic faces in congruent opposite pairs, ''C''''i'' symmetry, Order (group theory), order 2. Four points forming non-adjacent vertices of a rhombohedron necessarily form the four vertices of an orthocentric tetrahedron, and all orthocentric tetrahedra can be formed in this way. Rhombohedral lattice system The rhombohedral lattice system has rhombohedral cells, with 6 congruent rhombic faces forming a trigonal trapezohedron: : Special cases by symmetry * Cube: with Octahedral symmetry, Oh symm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal Family
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravais la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-stoichiometric Compound
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work. Contrary to earlier definitions, modern understanding of non-stoichiometric compounds view them as homogeneous, and not mixtures of stoichiometric chemical compounds. Since the solids are overall electrically neutral, the defect is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge. Many metal oxides and sulfides have non-stoichiometric examples; for example, stoichiometric iron(II) oxide, which is rare, has the formula , whereas the more common material is nonstoichi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molten Salt Battery
Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehicles and for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines. History Thermal batteries originated during World War II when German scientist Georg Otto Erb developed the first practical cells using a salt mixture as an electrolyte. Erb developed batteries for military applications, including the V-1 flying bomb and the V-2 rocket, and artillery fuzing systems. None of these batteries entered field use during the war. Afterwards, Erb was interrogated by British intelligence. His work was reported in "The Theory and Pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium–sulfur Battery
A sodium–sulfur battery is a type of molten-salt battery constructed from liquid sodium (Na) and sulfur (S). This type of battery has a high energy density (its energy density is 5 times that of a lead-acid battery), high efficiency of charge/discharge and is fabricated from inexpensive and non-toxic materials. The operating temperatures of 300 to 350 °C and the highly corrosive nature of the sodium polysulfides, primarily make them suitable for stationary energy storage applications. The cell becomes more economical with increasing size. Commercially available cells are typically large with high capacities (up to 500Ah). This is because of the Square-cube law: large cells have less relative heat loss, so maintaining their high operating temperatures is easier. These batteries, although having a reasonably long long cycle life (>1000 on average) are prone to disastrous failures due to a reaction between molten sodium and molten sulfur , and primarily for this reasons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Vehicle
An electric vehicle (EV) is a vehicle that uses one or more electric motors for propulsion. It can be powered by a collector system, with electricity from extravehicular sources, or it can be powered autonomously by a battery (sometimes charged by solar panels, or by converting fuel to electricity using fuel cells or a generator). EVs include, but are not limited to, road and rail vehicles, surface and underwater vessels, electric aircraft and electric spacecraft. For road vehicles, together with other emerging automotive technologies such as autonomous driving, connected vehicles and shared mobility, EVs form a future mobility vision called Connected, Autonomous, Shared and Electric (CASE) Mobility. EVs first came into existence in the late 19th century, when electricity was among the preferred methods for motor vehicle propulsion, providing a level of comfort and ease of operation that could not be achieved by the gasoline cars of the time. Internal combustion engin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |