|
Beta-Tungsten
Beta-tungsten (β-W) is a metastable phase of tungsten widely observed in tungsten thin films. While the commonly existing stable α-W has a body-centered cubic ( A2) structure, β-W adopts the topologically close-packed A15 structure containing eight atoms per unit cell, and it irreversibly transforms to the stable α phase through thermal annealing of up to 650°C. It has been found that β-W possesses the giant spin Hall effect, wherein the applied charge current generates a transverse spin current, and this leads to potential applications in magnetoresistive random access memory devices. History β-W was first observed by Hartmann et al. in 1931 as part of the dendritic metallic deposit formed on the cathode after electrolysis of phosphate melts below 650°C. In the beginning stages of research into β-W, oxygen was commonly found to promote the formation of the β-W structure, thus discussions of whether the β-W structure is a phase of single-element tungsten or a tung ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strukturbericht Designation
In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as supplement to space group crystal structures designations, especially historically. Each Strukturbericht designation is described by a single space group, but the designation includes additional information about the positions of the individual atoms, rather than just the symmetry of the crystal structure. While Strukturbericht symbols exist for many of the earliest observed and most common crystal structures, the system is not comprehensive, and is no longer being updated. Modern databases such as Inorganic Crystal Structure Database index thousands of structure types directly by the prototype compound (i.e. "the NaCl structure" instead of "the B1 structure"). These are essentially equivalent to the old Stukturbericht designations. History T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
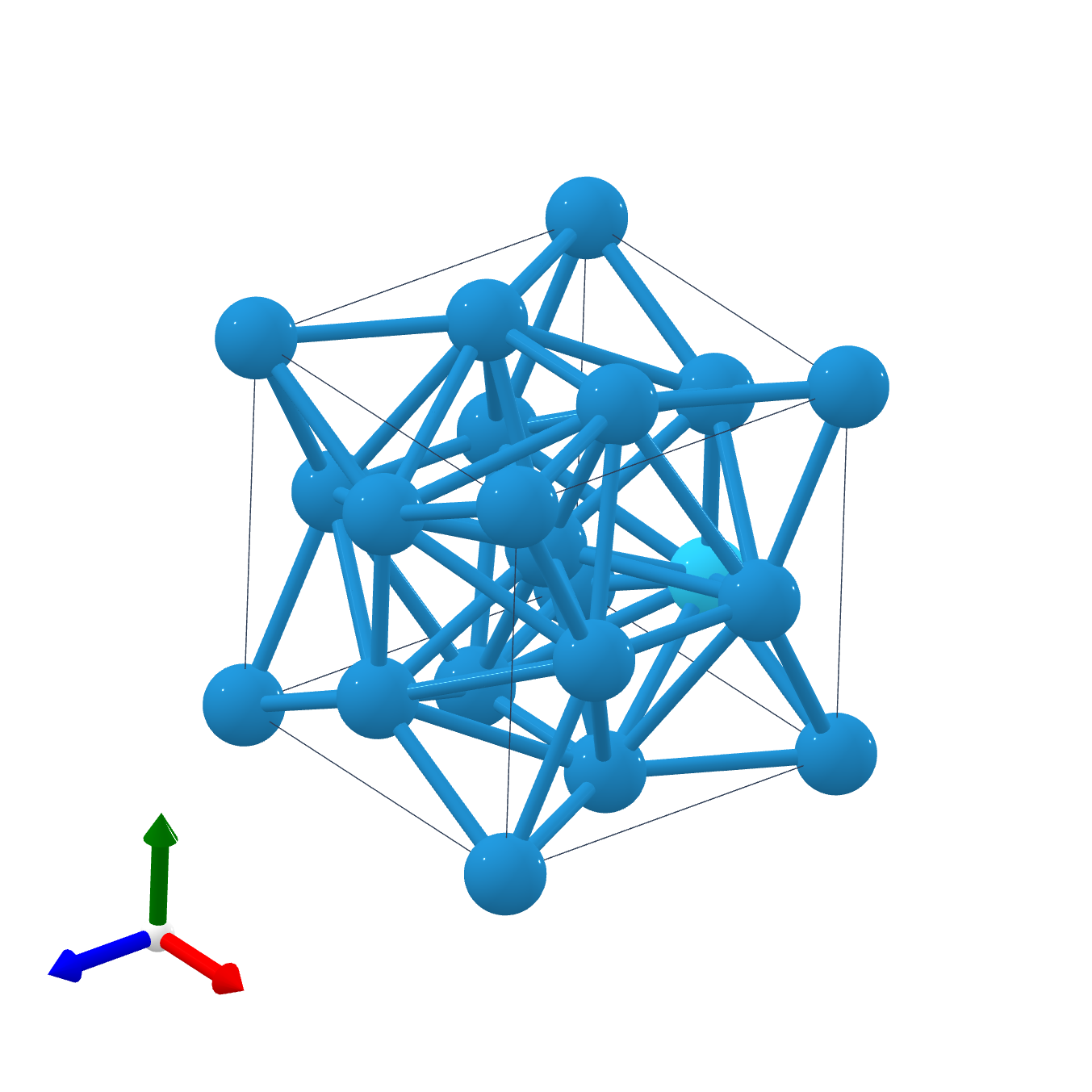
A15 Phases
The A15 phases (also known as β-W or Cr3Si structure types) are series of intermetallic compounds with the chemical formula ''A''3''B'' (where A is a transition metal and B can be any element) and a specific structure. The A15 phase is also one of the members in the Frank–Kasper phases family. Many of these compounds have superconductivity at around , which is comparatively high, and remain superconductive in magnetic fields of tens of teslas (hundreds of kilogauss). This kind of superconductivity ( Type-II superconductivity) is an important area of study as it has several practical applications. History The first time that A15 structure was observed was in 1931 when an electrolytically deposited layer of tungsten was examined. Discussion of whether the β-tungsten structure is an allotrope of tungsten or the structure of a tungsten suboxide was long-standing, but since the 1950s there has been many publications showing that the material is a true allotrope of tungsten. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Vapor Deposition
Physical vapor deposition (PVD), sometimes called physical vapor transport (PVT), describes a variety of vacuum deposition methods which can be used to produce thin films and coatings on substrates including metals, ceramics, glass, and polymers. PVD is characterized by a process in which the material transitions from a condensed phase to a vapor phase and then back to a thin film condensed phase. The most common PVD processes are sputtering and evaporation. PVD is used in the manufacturing of items which require thin films for optical, mechanical, electrical, acoustic or chemical functions. Examples include semiconductor devices such as thin-film solar cells, microelectromechanical devices such as thin film bulk acoustic resonator, aluminized PET film for food packaging and balloons, and titanium nitride coated cutting tools for metalworking. Besides PVD tools for fabrication, special smaller tools used mainly for scientific purposes have been developed. The source material i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
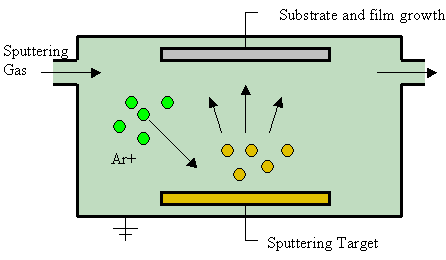
Sputter Deposition
Sputter deposition is a physical vapor deposition (PVD) method of thin film deposition by the phenomenon of sputtering. This involves ejecting material from a "target" that is a source onto a "substrate" such as a silicon wafer. Resputtering is re-emission of the deposited material during the deposition process by ion or atom bombardment. Sputtered atoms ejected from the target have a wide energy distribution, typically up to tens of eV (100,000 K). The sputtered ions (typically only a small fraction of the ejected particles are ionized — on the order of 1 percent) can ballistically fly from the target in straight lines and impact energetically on the substrates or vacuum chamber (causing resputtering). Alternatively, at higher gas pressures, the ions collide with the gas atoms that act as a moderator and move diffusively, reaching the substrates or vacuum chamber wall and condensing after undergoing a random walk. The entire range from high-energy ballistic impact to low-energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
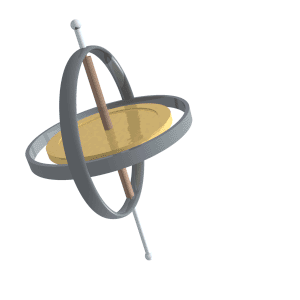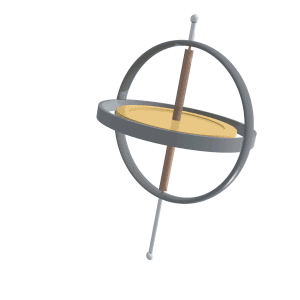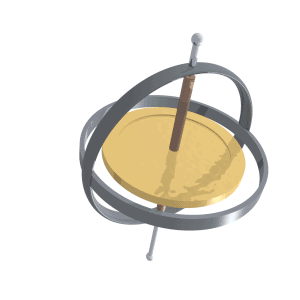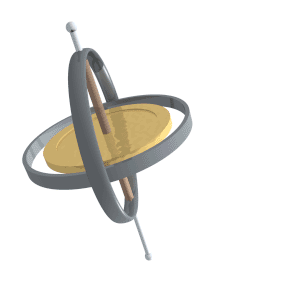
Precession
Precession is a change in the orientation of the rotational axis of a rotating body. In an appropriate reference frame it can be defined as a change in the first Euler angle, whereas the third Euler angle defines the rotation itself. In other words, if the axis of rotation of a body is itself rotating about a second axis, that body is said to be precessing about the second axis. A motion in which the second Euler angle changes is called ''nutation''. In physics, there are two types of precession: torque-free and torque-induced. In astronomy, ''precession'' refers to any of several slow changes in an astronomical body's rotational or orbital parameters. An important example is the steady change in the orientation of the axis of rotation of the Earth, known as the precession of the equinoxes. Torque-free Torque-free precession implies that no external moment (torque) is applied to the body. In torque-free precession, the angular momentum is a constant, but the angular velocit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Diametric. The origin of the magnetic moments responsible for magnetization can be either microscopic electric currents resulting from the motion of electrons in atoms, or the spin of the electrons or the nuclei. Net magnetization results from the response of a material to an external magnetic field. Paramagnetic materials have a weak induced magnetization in a magnetic field, which disappears when the magnetic field is removed. Ferromagnetic and ferrimagnetic materials have strong magnetization in a magnetic field, and can be ''magnetized'' to have magnetization in the absence of an external field, becoming a permanent magnet. Magnetization is not necessarily uniform within a material, but may vary between different points. Magnetizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin–orbit Interaction
In quantum physics, the spin–orbit interaction (also called spin–orbit effect or spin–orbit coupling) is a relativistic interaction of a particle's spin with its motion inside a potential. A key example of this phenomenon is the spin–orbit interaction leading to shifts in an electron's atomic energy levels, due to electromagnetic interaction between the electron's magnetic dipole, its orbital motion, and the electrostatic field of the positively charged nucleus. This phenomenon is detectable as a splitting of spectral lines, which can be thought of as a Zeeman effect product of two relativistic effects: the apparent magnetic field seen from the electron perspective and the magnetic moment of the electron associated with its intrinsic spin. A similar effect, due to the relationship between angular momentum and the strong nuclear force, occurs for protons and neutrons moving inside the nucleus, leading to a shift in their energy levels in the nucleus shell model. In the fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resistor
A resistor is a passive two-terminal electrical component that implements electrical resistance as a circuit element. In electronic circuits, resistors are used to reduce current flow, adjust signal levels, to divide voltages, bias active elements, and terminate transmission lines, among other uses. High-power resistors that can dissipate many watts of electrical power as heat may be used as part of motor controls, in power distribution systems, or as test loads for generators. Fixed resistors have resistances that only change slightly with temperature, time or operating voltage. Variable resistors can be used to adjust circuit elements (such as a volume control or a lamp dimmer), or as sensing devices for heat, light, humidity, force, or chemical activity. Resistors are common elements of electrical networks and electronic circuits and are ubiquitous in electronic equipment. Practical resistors as discrete components can be composed of various compounds and forms. Resisto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Resistivity And Conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). For example, if a solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is , then the resistivity of the material is . Electrical conductivity or specific conductance is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter ( sigma), but (kappa) (especially in electrical engineering) and (gamma) are sometimes used. The SI unit of electrical conductivity is siemens per metre (S/m). Resistivity and conductivity are intensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)