|
Bestatin
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from ''Streptomyces olivoreticuli''. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds. See also * Amastatin * Pepstatin References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amastatin
Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from ''Streptomyces sp. ME 98-M3''. It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase (aminopeptidase M/N), bacterial leucyl aminopeptidase (''Aeromonas proteolytica'' aminopeptidase), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and, to a lesser extent, glutamyl aminopeptidase (aminopeptidase A), as well as other aminopeptidases. It does not inhibit arginyl aminopeptidase (aminopeptidase B). Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin ''in vivo''. It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides. See also * Bestatin * Pepstatin Pepstatin is a potent inhibitor of aspartyl proteases. It is a hexa-peptide containing the unusual amino acid statine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
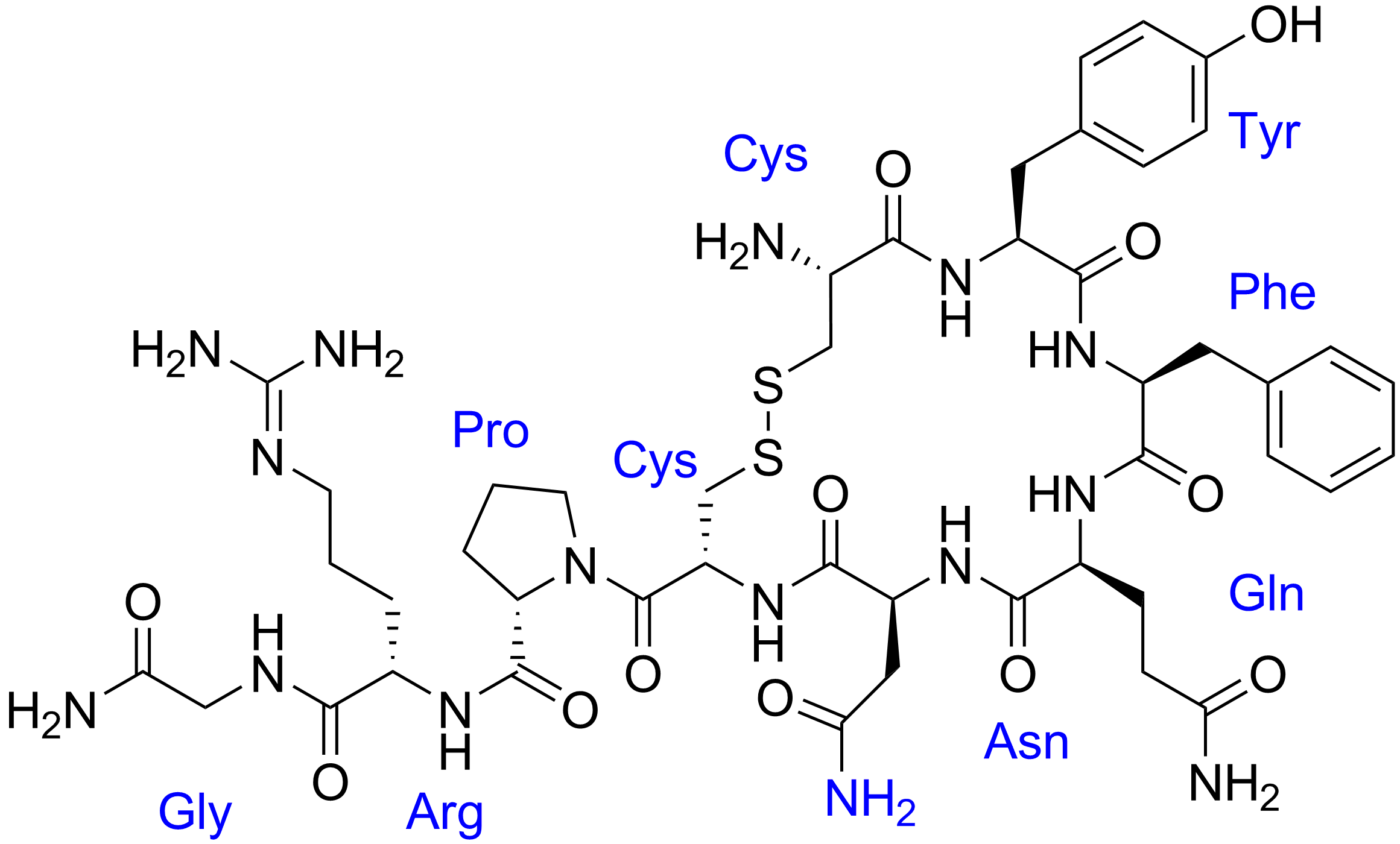
Pepstatin
Pepstatin is a potent inhibitor of aspartyl proteases. It is a hexa-peptide containing the unusual amino acid statine (Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid), having the sequence Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta-Ala-Sta). It was originally isolated from cultures of various species of Actinomyces due to its ability to inhibit pepsin at picomolar concentrations. Pepstatin A is well known to be an inhibitor of aspartic proteases such as pepsin, cathepsins D and E. Except for its role as a protease inhibitor, however, the pharmacological action of pepstatin A upon cells remain unclear. Pepstatin A suppresses receptor activator of NF-κB ligand (RANKL)–induced osteoclast differentiation. Pepstatin A suppresses the formation of multinuclear osteoclasts dose-dependently. This inhibition of the formation only affected osteoclast cells, i.e., not osteoblast-like cells. Furthermore, pepstatin A also suppresses differentiation from pre-osteoclast cells to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymphedema
Lymphedema, also known as lymphoedema and lymphatic edema, is a condition of localized swelling caused by a compromised lymphatic system. The lymphatic system functions as a critical portion of the body's immune system and returns interstitial fluid to the bloodstream. Lymphedema is most frequently a complication of cancer treatment or parasitic infections, but it can also be seen in a number of genetic disorders. Though incurable and progressive, a number of treatments may improve symptoms. Tissues with lymphedema are at high risk of infection because the lymphatic system has been compromised. While there is no cure, treatment may improve outcomes. This commonly include compression therapy, good skin care, exercise, and manual lymphatic drainage (MLD), which together are known as combined decongestive therapy. Diuretics are not useful. Signs and symptoms The most common manifestation of lymphedema is soft tissue swelling, edema. As the disorder progresses, worsening edema ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease Inhibitors
Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS and hepatitis C. These protease inhibitors prevent viral replication by selectively binding to viral proteases (e.g. HIV-1 protease) and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles. Protease inhibitors that have been developed and are currently used in clinical practice include: * Antiretroviral HIV-1 protease inhibitors—class stem ** Amprenavir ** Atazanavir ** Darunavir ** Fosamprenavir ** Indinavir ** Lopinavir ** Nelfinavir ** Ritonavir ** Saquinavir ** Tipranavir * Hepatitis C virus NS3/ 4A protease inhibitors—class stem ** Asunaprevir ** Boceprevir ** Grazoprevir ** Glecaprevir ** Paritaprevir ** Simeprevir ** Telaprevir * Severe acute respiratory syndrome coronavirus 2 3-chymotrypsin-like p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enkephalinase Inhibitors
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include: * Aminopeptidase N (APN) * Neutral endopeptidase (NEP) * Dipeptidyl peptidase 3 (DPP3) * Carboxypeptidase A6 (CPA6) * Leucyl/cystinyl aminopeptidase (LNPEP) * Angiotensin-converting enzyme Angiotensin-converting enzyme (), or ACE, is a central component of the renin–angiotensin system (RAS), which controls blood pressure by regulating the volume of fluids in the body. It converts the hormone angiotensin I to the active vasoconstr ... (ACE) See also * Enkephalinase inhibitor * Oxytocinase References Hydrolases Human proteins Nociception {{Enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEROPS
MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitors by Rawlings ''et al.'' in 2004.Rawlings, N.D., Tolle, D.P. & Barrett, A.J. (2004) "Evolutionary families of peptidase inhibitors." ''Biochem J'' 378, 705-716. The most recent version, MEROPS 12.3, was released in September 2020. Overview The classification is based on similarities at the tertiary and primary structural levels. Comparisons are restricted to that part of the sequence directly involved in the reaction, which in the case of a peptidase must include the active site, and for a protein inhibitor the reactive site. The classification is hierarchical: sequences are assembled into families, and families are assembled into clans. Each peptidase, family, and clan has a unique identifier. Classification Family The families of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enkephalin
An enkephalin is a pentapeptide involved in regulating nociception in the body. The enkephalins are termed endogenous ligands, as they are internally derived and bind to the body's opioid receptors. Discovered in 1975, two forms of enkephalin have been found, one containing leucine ("leu"), and the other containing methionine ("met"). Both are products of the proenkephalin gene. * Met-enkephalin is Tyr-Gly-Gly-Phe-Met. * Leu-enkephalin has Tyr-Gly-Gly-Phe-Leu. Endogenous opioid peptides There are three well-characterized families of opioid peptides produced by the body: enkephalins, B-endorphin, and dynorphins. The met-enkephalin peptide sequence is coded for by the enkephalin gene; the leu-enkephalin peptide sequence is coded for by both the enkephalin gene and the dynorphin gene. The proopiomelanocortin gene ( POMC) also contains the met-enkephalin sequence on the N-terminus of beta-endorphin, but the endorphin peptide is not processed into enkephalin. Effects on stre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vasopressin
Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity ( hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure. A third function is possible. Some AVP may be released directly into the brain from the hypothalamus, and may play an important role in social behavior, sexual motivation and pair bonding, and maternal responses to stress. Vasopressin induces differentiation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxytocin
Oxytocin (Oxt or OT) is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. It plays a role in social bonding, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during labour. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in bonding with the baby and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |