|
BMS-F
BMS-F is a chemical from the aminoalkylindole family invented by Bristol-Myers Squibb around 1999, that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 8 nM at CB2 and 500x selectivity over the related CB1 receptor. It has antiinflammatory effects and inhibits release of TNF-α. See also * A-796,260 * APP-FUBINACA * JWH-200 * MDMB-FUBINACA * MN-25 * Pravadoline * S-777,469 * WIN 55,212-2 WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure. WIN 55,212-2 is a potent canna ... References {{Cannabinoids Aminoalkylindoles Designer drugs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A-796,260
A-796,260 is a drug developed by Abbott Laboratories that acts as a potent and selective cannabinoid CB2 receptor agonist. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3- tetramethylcyclopropyl methanone group, imparts significant selectivity for CB2, and A-796,260 was found to be a highly selective CB2 agonist with little affinity for CB1, having a CB2 ''K''i of 4.6 nM vs 945 nM at CB1. It has potent analgesic and anti-inflammatory actions in animal models, being especially effective in models of neuropathic pain, but without producing cannabis-like behavioral effects. Legal Status As of October 2015 A-796,260 is a controlled substance in China. See also * A-834,735 * A-836,339 * BMS-F * JWH-200 * UR-144 * XLR-11 XLR-11 (5"-fluoro-UR-144 or 5F-UR-144) is a drug that acts as a potent agonist for the cannabinoid receptors CB1 and CB2 with EC50 values of 98 nM and 83 nM, respectively. It is a 3-(tetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
APP-FUBINACA
APP-FUBINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. Pharmacological testing showed APP-FUBINACA to have only moderate affinity for the CB1 receptor, with a Ki of 708 nM, while its EC50 was not tested. It contains a phenylalanine amino acid residue in its structure. Legality Sweden's public health agency suggested to classify APP-FUBINACA as hazardous substance on March 24, 2015. See also * 5F-AB-PINACA * 5F-ADB * 5F-AMB * 5F-APINACA * AB-FUBINACA * AB-CHFUPYCA * AB-CHMINACA * AB-PINACA * ADB-CHMINACA * ADB-FUBINACA * ADB-PINACA * ADBICA * APICA * APINACA * APP-BINACA * BMS-F * MDMB-CHMICA * MDMB-FUBINACA * PX-1 * PX-2 * PX-3 PX-3 (also known as APP-CHMINACA) is an indazole-based synthetic cannabinoid. It is a potent agonist of the CB1 receptor with a binding affinity of ''K''i = 47.6 nM and was originally developed by Pfizer in 2009 as an analgesic m ... References Cannabinoids Designer drugs Ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-777,469
S-777,469 is a drug developed by Shionogi which is a cannabinoid receptor agonist, with 128x selectivity for the CB2 subtype, having a CB2 affinity of 36nM, and a CB1 affinity over 4600nM. In animal studies it showed antipruritic effects, and passed Phase II human trials for the treatment of atopic dermatitis, but development was ultimately not continued further. See also * BMS-F * RQ-00202730 * S-444,823 S-444,823 is a drug developed by Shionogi which is a cannabinoid agonist. It was developed as an antipruritic, and has moderate selectivity for the CB2 subtype, having a CB2 affinity of 18nM, and 32x selectivity over the CB1 receptor. In animal ... References Cannabinoids Nitrogen heterocycles {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MN-25
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a ''K''i of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a ''K''i of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a ''K''i of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098). Chemically, it is closely related to another indole-3-carboxamide synthetic cannabinoid, Org 28611, but with a different cycloalkyl substitution on the carboxamide, and the cyclohexylmethyl group replaced by morpholinylethyl, as in JWH-200 or A-796,260. Early compounds such as these have subsequently led to the development of many related indole-3-carboxamide cannabinoid ligands. See also * A-834,735 * AB-0 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-200
JWH-200 (WIN 55,225) is an analgesic chemical from the aminoalkylindole family that acts as a cannabinoid receptor agonist. Its binding affinity, ''K''i at the CB1 receptor is 42 nM, around the same as that of THC, but its analgesic potency ''in vivo'' was higher than that of other analogues with stronger CB1 binding affinity ''in vitro'', around 3 times that of THC but with less sedative effect, most likely reflecting favourable pharmacokinetic characteristics. It was discovered in 1991 by Sterling Drug as a potential analgesic following the earlier identification of related compounds such as pravadoline and WIN 55,212-2. Legal status Australia JWH-200 is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015). A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
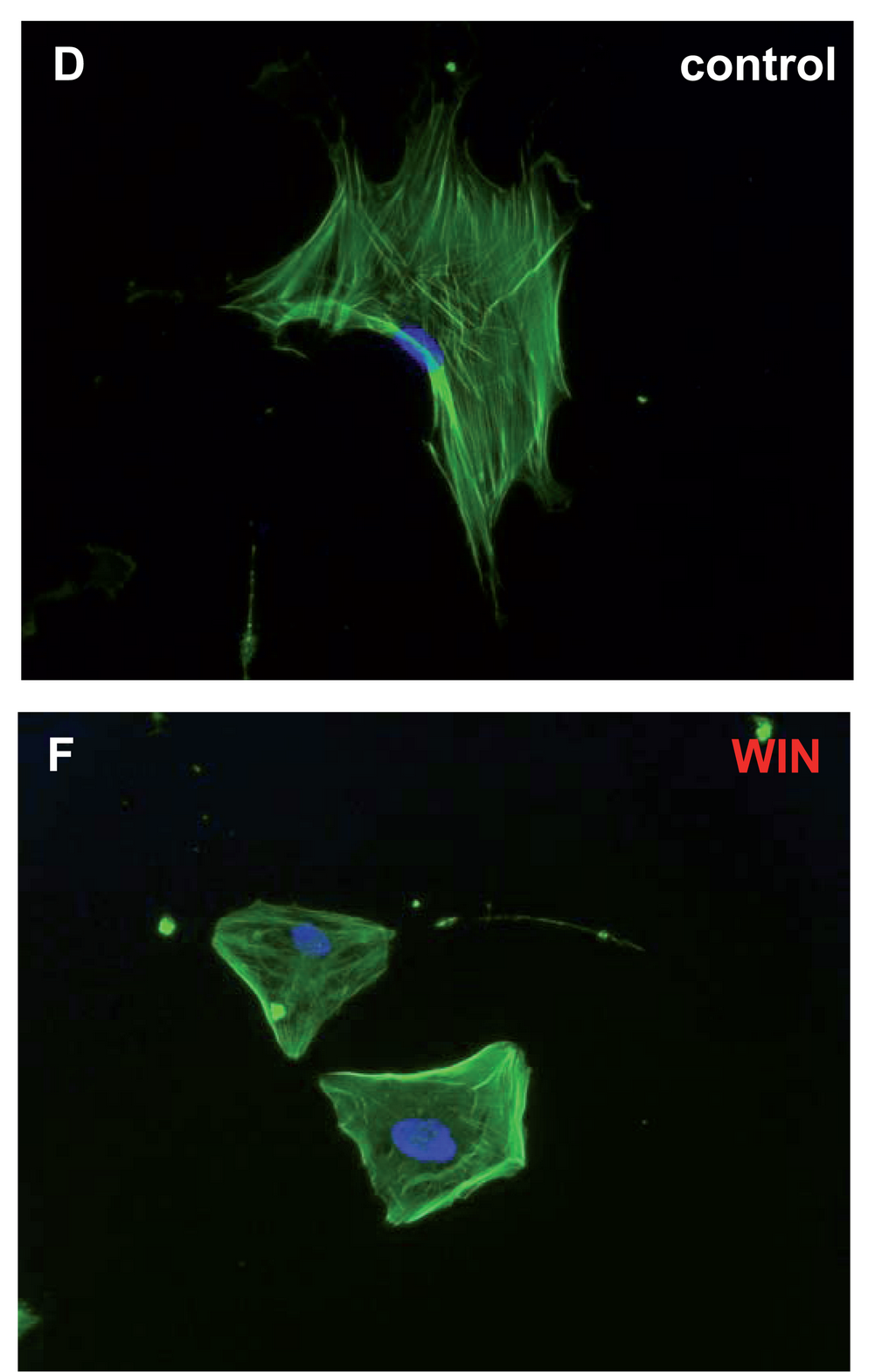
WIN 55,212-2
WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure. WIN 55,212-2 is a potent cannabinoid receptor agonist that has been found to be a potent analgesic in a rat model of neuropathic pain. It activates p42 and p44 MAP kinase via receptor-mediated signaling. At 5 μM WIN 55,212-2 inhibits ATP production in sperm in a CB1 receptor-dependent fashion. WIN 55,212-2, along with HU-210 and JWH-133, may prevent the inflammation caused by amyloid beta proteins involved in Alzheimer's disease, in addition to preventing cognitive impairment and loss of neuronal markers. This anti-inflammatory action is induced through agonist action at cannabinoid receptors, which prevents microglial activation that elicits the inflammation. WIN 55,212-2 is a full agonist at the CB1 cannabinoid receptor ( ''K''i = 1.9 nM) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pravadoline
Pravadoline (WIN 48,098) is an antinflammatory and analgesic drug with an IC50 of 4.9 μM and a ''K''i of 2511 nM at CB1, related in structure to nonsteroidal anti-inflammatory drugs (NSAIDs) such as indometacin. It was developed in the 1980s as a new antiinflammatory and prostaglandin synthesis inhibitor, acting through inhibition of the enzyme cyclooxygenase (COX). However, pravadoline was found to exhibit unexpectedly strong analgesic effects, which appeared at doses ten times smaller than the effective anti-inflammatory dose and so could not be explained by its action as a COX inhibitor. These effects were not blocked by opioid antagonists such as naloxone, and it was eventually discovered that pravadoline represented the first compound from a novel class of cannabinoid agonists, the aminoalkylindoles. Pravadoline was never developed for use as an analgesic, partly due to toxicity concerns (although these were later shown to be a result of the salt form that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MDMB-FUBINACA
MDMB-FUBINACA (also known as MDMB(N)-Bz-F and FUB-MDMB) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with ''K''i values of 1.14 nM at CB1 and 0.1228 nM at CB2 and EC50 values of 0.2668 nM at CB1 and 0.1411 nM at CB2, and has been sold online as a designer drug. Its benzyl analogue (instead of 4-fluorobenzyl) has been reported to be a potent agonist for the CB1 receptor (''K''i = 0.14 nM, EC50 = 2.42 nM). The structure of MDMB-FUBINACA contains the amino aci3-methylvalineor tert-leucine methyl ester. Side effects There have been a large number of reported cases of deaths and hospitalizations in relation to this synthetic cannabinoid, mainly in Russia and Belarus. MDMB-FUBINACA was first reported in 2014 and quickly gained a reputation as the most deadly synthetic cannabinoid drug sold by 2015. Up to 700 hospitalisations and 25 deaths were initially linked to MDMB-FUBINACA in media and govern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoalkylindole
Aminoalkylindoles (AAIs) are a family of cannabinergic compound that act as a cannabinoid receptor agonist. They were invented by pharmaceutical company Sterling-Winthrop in the early 1990s as potential nonsteroidal anti-inflammatory agents. Legality Aminoalkylindoles are now commonly found in synthetic cannabis designer drugs. In the United States, the DEA added the aminoalkylindoles JWH-200 to Schedule I of the Controlled Substances Act on 1 March 2011 for 12 months. References {{reflist External links Aminoalkylindoles ChEBI Chemical Entities of Biological Interest, also known as ChEBI, is a chemical database and ontology of molecular entities focused on 'small' chemical compounds, that is part of the Open Biomedical Ontologies (OBO) effort at the European Bioinform ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bristol-Myers Squibb
The Bristol Myers Squibb Company (BMS) is an American multinational pharmaceutical company. Headquartered in New York City, BMS is one of the world's largest pharmaceutical companies and consistently ranks on the ''Fortune'' 500 list of the largest U.S. corporations. For fiscal 2021, it had a total revenue of $46.4 billion. Bristol Myers Squibb manufactures prescription pharmaceuticals and biologics in several therapeutic areas, including cancer, HIV/AIDS, cardiovascular disease, diabetes, hepatitis, rheumatoid arthritis, and psychiatric disorders. BMS's primary research and development (R&D) sites are located in Lawrence, New Jersey (formerly Squibb, near Princeton), Summit, New Jersey, formerly HQ of Celgene, New Brunswick, New Jersey, Redwood City, California, and Seville in Spain, with other sites in Devens and Cambridge, Massachusetts, East Syracuse, New York, Braine-l'Alleud, Belgium, Tokyo, Japan, Bangalore, India, and Wirral, United Kingdom. BMS previously had ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TNF-α
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain. As an adipokine, TNF promotes insulin resistance, and is associated with obesity-induced type 2 diabetes. As a cytokine, TNF is used by the immune system for cell signaling. If macrophages (certain white blood cells) detect an infection, they release TNF to alert other immune system cells as part of an inflammatory response. TNF signaling occurs through two receptors: TNFR1 and TNFR2. TNFR1 is constituitively expressed on most cell types, whereas TNFR2 is restricted primarily to endothelial, epithelial, and subsets of immune cells. TNFR1 signaling tends to be pro-inflammatory and apoptotic, whereas TNFR2 signaling is anti-inflammatory and promotes cell proliferation. Suppression of TNFR1 signaling has been important fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiinflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs remedy pain by reducing inflammation as opposed to opioids, which affect the central nervous system to block pain signaling to the brain. Nonsteroidal anti-inflammatory drugs Nonsteroidal anti-inflammatory drugs (NSAIDs) alleviate pain by counteracting the cyclooxygenase (COX) enzyme. On its own, COX enzyme synthesizes prostaglandins, creating inflammation. In whole, the NSAIDs prevent the prostaglandins from ever being synthesized, reducing or eliminating the inflammation and resulting pain. Some common examples of NSAIDs are aspirin, ibuprofen, and naproxen. The newer specific COX-inhibitors are not classified together with the traditional NSAIDs, even though they presumably share the same mode of action. On the other hand, there are analgesics that are commonly associat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)